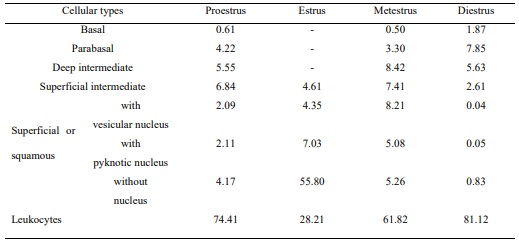
Table 1. Distribution of the cellular types at the stages of the estrous cycle in M. c. bonariensis. Values expressed as percentages (%).
ARTÍCULO DE INVESTIGACIÓN
Qualitative and quantitative variations of the vaginal epithelium in Myocastor coypus bonariensis (coypu) during the estrous cycle
Felipe, AE1; Lombardo, DM2,3,4.
1
Área de Ciencias Morfológicas, Facultad de Ciencias Veterinarias, UNCPBA, Campus Universitario, Tandil (7000).
2
Universidad de
Buenos Aires Facultad de Ciencias Veterinarias, Instituto de Investigación y Tecnología en Reproducción Animal (INITRA), Buenos
Aires, Argentina.
3
Universidad de Buenos Aires, Facultad de Ciencias Veterinarias, Cátedra de Histología y Embriología, Buenos
Aires, Argentina
4
CONICET, Buenos Aires, Argentina.
Recibido:14/06/2021
Aceptado:14/02/2022
Correspondencia e-mail:Antonio E. Felipe aefelipe@vet.unicen.edu.ar
Resumen
El objetivo del presente estudio fue caracterizar los cambios cualitativos y cuantitativos en el epitelio vaginal del coipo (M. c. Bonariensis) durante el ciclo estral. Se realizó una evaluación colpocitológica diaria en 36 hembras. Las muestras de tres zonas vaginales (cefálica, media y caudal) se procesaron con técnicas histológicas de rutina. La duración media del ciclo fue de 36,86 ± 10,52 días. El mayor grosor del epitelio se observó en el proestro y el menor en el metaestro. Las variaciones en el espesor epitelial en cada etapa del ciclo para las diferentes regiones muestreadas no mostraron diferencias significativas en estro y proestro. Se observaron diferencias durante el metaestro entre las regiones cefálica y media y media y caudal, y también durante el diestro, siendo las más significativas entre las regiones cefálica y media. En el metaestro, se observó una disminución significativa en el número de capas epiteliales en todas las regiones. En el diestro se observaron diferencias entre las regiones cefálica y media y cefálica y caudal.
Palabras clave:coipo, Myocastor coypus, roedores histricognatos, vagina, ciclo estral
Variaciones cualitativas y cuantitativas del epitelio vaginal en Myocastor coypus bonariensis (coipo) durante el ciclo estral
ABSTRACT
The aim of the present study was to characterize the qualitative and quantitative changes in the vaginal epithelium in the coypu (M. c. bonariensis) during the estrous cycle. A daily colpocytological evaluation was performed in 36 females. Samples from three vaginal sections (cephalic, middle and caudal) were processed with routine histological techniques. The mean cycle duration was of 36.86 ± 10.52 days. The greater thickness of the epithelium was observed in the proestrous and the smallest in the metestrous. Variations in epithelial thickness in each cycle stage for the different sampled regions showed nonsignificant differences in the estrous and proestrous. Differences were observed during the metestrous between the cephalic and middle and middle and caudal regions, and also during the diestrous, being the most significant between cephalic and middle regions. At metestrous, a significant decrease in the number of epithelial layers was observed in all the regions. At diestrous, differences were observed between cephalic and middle, and cephalic and caudal regions.
Key words:coypu, Myocastor coypus, hystricognath rodents, vagina, estrous cycle
INTRODUCCIÓN
According to Ojasti’s14 classification criteria of the wild fauna managing, the coypu is classified as a farming species which its native population is able to be used. Other South American hystricognath rodents of value for economical resources are Hydrochoerus hydrochoeris (capibara), Dasyprocta aguti (aguti), Agouti paca (pacas), Cavia porcellus (cuy or guinea pig), Lagostomus maximus (prairie viscacha), Proechimys guairae (casiragua or spinous rat or guaira spiny rat) and Chinchilla laniger (chinchilla). Most of the hystricognath, as the coypu, presents some differential characteristics from other groups of rodents, such as courtship activity, long gestation periods, delivery of precocious offspring and the presence of a vaginal occlusion membrane. In the coypu, the complete absence of this membrane once sexual maturity is reached has allowed colpocytological studies of the estrous cycle7 . Researches on the reproductive biology of the coypu have been performed on wild natural populations4 , under conditions of semi-captivity, at commercial farming or in experimental populations7. The reproductive system of the coypu has been anatomically and histologically characterized5,6, however to our knowledge there are not information regarding its functional modifications under different physiological conditions. Among these modifications, morphofunctional variations during the estrous cycle are of great importance. Cyclic changes in the reproductive tract have been reported in many domestic and laboratory species15. The colpocytological studies performed on the coypu have corroborated the existence of a typical estrous cycle, rendering it as an annual polyestrous rodent. The normal duration of its estrous cycle is 35.5 ± 10.8 days, ranking from 20 to 60 days7 . The aim of the present study was to characterize the changes in the vaginal epithelium during the estrous cycle of M. c. bonariensis by using a qualitative and quantitative morphological analysis, taking into account macroscopic aspects of the mucosa, thickness of the vaginal epithelium, number of cellular layers and types of superficial cells.
MATERIALS AND METHODS
Thirty six (36) virgin and sexually mature
females of the subspecies M. c.bonariensis were used.
Females, born in captivity, were kept under herding
conditions located in parlors with the presence of a
male of the same subspecies in an adjacent parlor.
At the time of sacrifice, the animals were 23 months
old and weighed 5.42 ± 0.31 kg. The colpocytological
follow-up of the animals was carried out daily for
18 months from 5 month of age in order to obtain
records of 7 complete estrous cycles per animal.
Vaginal smears were observed as collected within
5 minutes of sampling and after its staining with
hematoxilin and Shorr dye. To determine the stage
of the cycle, the cytological composition of vaginal
smears was considered as described by Felipe et
al.
7
. Determination of the number of each cellular
type identified was carried out by using a lattice of
known dimensions located in a microscope with screen. A strategy of random sampling was used in
the counting of cells. For each smear, a total of 30
fields were counted with a 10x ocular. The length
of each estrous cycle was considered as the interval
between the first day of estrous, determined by
colpocytology, and the day before to the next
estrous. To determine regional variations of the
characteristics of the vaginal epithelium, the organ
was divided into three sections: cephalic or of the
vaginal fornix, middle (between the anal sac and
the urinary bladder) and caudal. Samples were
fixed in Bouin´s liquid and processed with routine
techniques and then embedded in paraffin. Serial
cuts of 5 mm were performed and stained with
Harris’ hematoxilin and eosin. Ten sections of each
region were examined to determining the thickness
of the epithelium and the number of epithelial
layers. In both cases, considering the ovoidal shape
of the vaginal lumen, measures and counting were
taken in four areas of the vaginal wall (dorsal, right
and left laterals and ventral). Measures were done
using a micrometric ocular of 100x attached to an
Olympus CH2 microscope. Counting of the number
of layers was done with a 400x.
Two serial cuts (one previous to and
one after the other ten cuts used for the above
mentioned determinations) were stained with
Harris’ hematoxilin and Shorr dye to facilitate the
identification of the different types of superficial
cells in each area for each stage of the estrous cycle.
Classification of the cellular type was performed
based on previous results7
, considering cells as
superficial or squamous, superficial intermediate,
deep intermediate, parabasal and basal. Values
obtained were expressed as percentage of each
cellular type per vaginal section. Statistical
analysis of the data was done with GraphPad
InStat software, version 3.0. Data are presented as
mean ± standard deviation (S.D.) and the level of
significance was always P<0.05.
RESULTS
Cellular characterization of the stages of the estrous cycle
During the proestrous, smears were of different types of epithelial cells, which were alone or in groups, mixed with abundant leukocytes (Table 1). The observed basal and parabasal cells in the proestrous showed a marked acidophile in their cytoplasms. The intermediate cells presented a basophilous cytoplasm o lightly acidophilus and a vesicular nucleus. The superficial cells were translucent and basophilic. The samples collected during the estrous showed abundance of squamous cells, strongly eosinophilic and arranged as aggregates, with few leukocytes and other cellular types (Table 1). In the metestrous, the presence of basal, parabasal and intermediate cells mixed with cornified cells and a marked increment of leukocytes was observed. A predominance of leukocytes, either alone or in groups, was observed in the diestrous, as well as filaments of mucous aspect. The predominance of leukocytes in vaginal samples was evident not only at the diestrous and metestrous but also at the proestrous (Table 1).

Table 1. Distribution of the cellular types at the stages of the estrous cycle in M. c. bonariensis. Values expressed as percentages (%).
Thickness of the epithelium
No statistically significant differences were observed in the thickness of the vaginal epithelium between samples of the proestrous and estrous; however, there were differences between samples of these stages and those of the metestrous and diestrous (P <0.001) (Figure 1). The greatest thickness of the epithelium was observed in the proestrous and the smallest thickness in the metestrus.
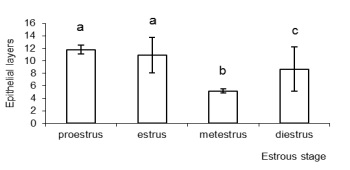
Figure 1.Variation in the thickness of the vaginal epithelium of M. c. bonariensis at different stages of the estrous
cycle. Bars with different letters are significantly different (P< 0.001).
The analysis of the epithelial thickness in the different regions during each stage showed no significant differences among them when comparing proestrous and estrous (Figure 2). However, differences were observed during metestrous between the cephalic and middle regions (P <0.05) and between the middle and caudal (P <0.001) regions. Also, there were differences during diestrous among the three regions, being more marked that between the cephalic and middle regions (P <0.001) (Figure 2). The comparison among regions of the vagina at different stages of the estrous cycle showed no significant differences in the proestrous and estrous among the cephalic, middle and caudal regions, but there were differences between regions in these two stages and the regions in the metestrous and diestrous (P <0.001).
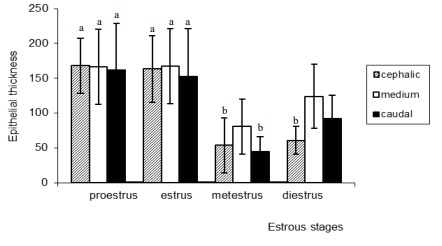
Figure 2.Variations in the epithelial thickness of M. c. bonariensis within each stage of the cycle considering the different
vaginal regions analyzed. Bars with different letters are significantly different (P < 0. 001, except b vs. c, P < 0.05).
Number of epithelial layers
The greatest number of layers was observed in the proestrous (Figure 3). The analysis of regional variations in number of layers within each stage of the cycle showed that in the proestrous there were no differences among regions. The decrease in the number of layers in the estrous was more marked in the caudal region (Figure 3), differences were significant when compared to the cephalic and middle regions (P <0.001). In the metestrous, a decrease in the number of epithelial layers was observed in all the regions (Figure 3), and the middle region presented significant differences compared to the cephalic and caudal regions (P <0.01). In the diestrous, differences were observed between cephalic and middle and cephalic and caudal regions (P <0.001). In the metestrous and estrous, the middle region showed a greater number of layers compared to the other two regions (cephalic and caudal regions) (Figure 3).
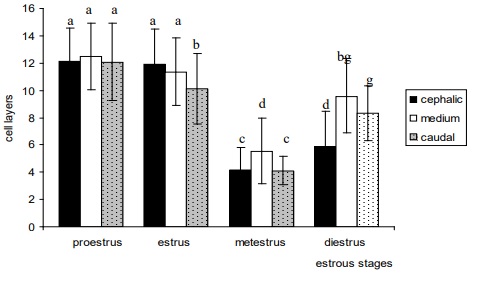
Figure 3.Number of epithelial cell layers in different stages of the estrous cycle and regions of the vagina of M. c.
bonariensis. Bars with different letters are significantly different (P < 0. 001, except c vs. d, P < 0.01).
Types of superficial cells during each stage of the estrous cycle
During the proestrous and estrous,
a predominance of superficial cells of the
squamous type in all the regions was observed
(Table 2). In the vaginal epithelium, during the
proestrous, a tendency to detachedness of the
more superficial layers of the epithelium was
observed. In the metestrous, the predominant
cells on the surface were of the intermediate
deep type in the middle and caudal regions and
intermediate superficial type in the cephalic
region. Both cellular types showed a cuboidal
shape with round or slightly ovoidal nuclei. In the
diestrous, samples presented a predominance of
intermediate superficial cells (Table 2).
Figure 4 shows microphotographs of the
cell types observed in colpocytological samples.
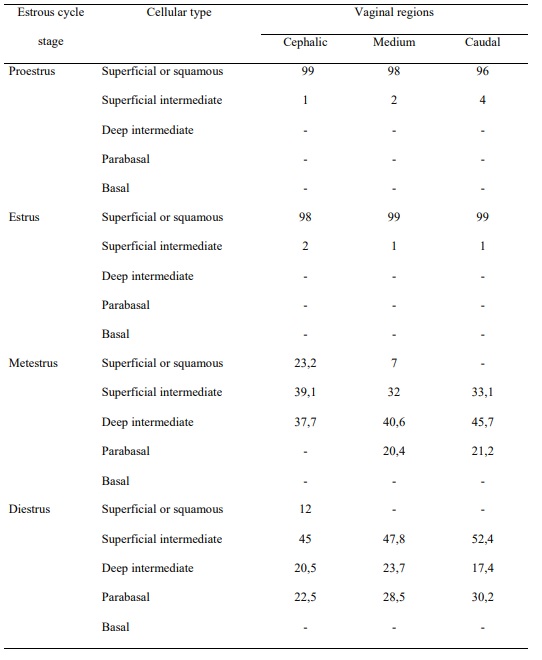
Table 2.Cell types in the different regions of the vagina and in different stages of the estrous cycle in M. c.
bonariensis. Values expressed as a percentage.
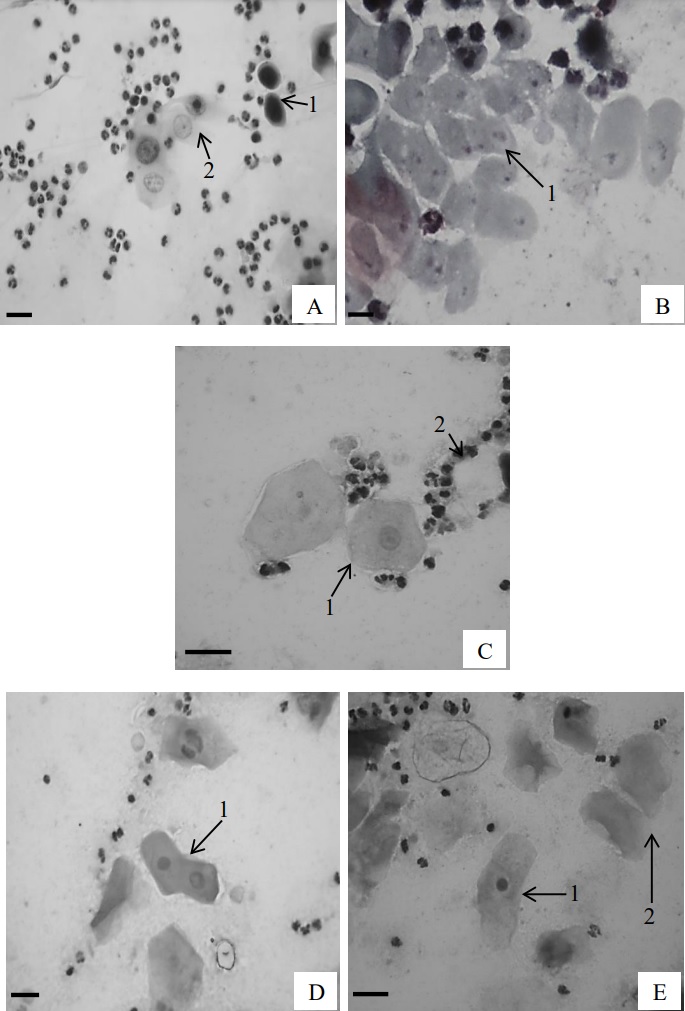
Figure 4.Cellular types observed in colpocytological samples. A.1- basal cell, A.2- parabasal cell; B.3- deep intermediate
cell; C.1- superficial intermediate cell, C.2- polymorphonuclear lymphocytes; D.1- superficial cells with vesicular
nucleus; E.1- superficial cell with pyknotic nucleus, E.2- superficial cells without nucleus. Shorr´s stain. Bar: 10 µm.
DISCUSSION
Results of the present study indicate
that the estrous cycle of M. c. bonariensis
is characterized by its long duration. The
prolonged duration of the estrous cycles of
the coypu is common in the members of the
Suborder Histricognathi9,11,13. In the cavies, such
as Cavia porcellus, the cycle lasts 16.5 days1
and
in Cavia aperea 20.6 days. In Galea musteloides,
it lasts 22.3 days23, in Chinchilla laniger, 38.1
days18, 20 and in Lagostomus maximus, 45 days21,
22. Conversely, in the rodents of the Suborder
Miomorpha, estrous cycles are relatively short.
For example, it lasts 5 days in the mouse, 4
to 6 days in the Mongolian gerbil (Meriones
unguiculatus)2
, 5 to 6 days in the rat22 and from
8 to 9 days in Oryzomys y Sigmodon. In rodents
of the Suborder Sciurognathi, such as Cricetomys
gambianus, the mean duration of the cycle is 7.9
days, ranging from 3 to 15 days10, being more
prolonged in Pectinator spekei (22.7 days) and in
Ctenodactylus gundi (23 to 25 days)11.
The composition of the vaginal smears
from the coypu showed a typical sequence of
rodents. The successive dominance of leukocytes,
nucleated epithelial cells and cornified cells has
been described in murine rodents such as the
hamster15, mouse15, gerbil2
, rat19 and Calomys
callosus16, and in a hystricognath such as the
guinea pig8,23, Cavia aperea, Galea musteloides17,
and Atherurus africanus11. These changes are
directly associated with the modifications in the
vaginal epithelium, for example the variations in
the type of superficial cells, mitosis and apoptosis
indexes, the keratinization, the increase of the
thickness and the number of cellular layers and
the leukocytic infiltration16.
Besides, the qualitative and quantitative
changes observed during the estrous cycle in
the coypu are similar to those described for
other rodent species. Weir20, 21 reported that,
in Dasyprocta agouti and Myoprocta pratti,
morphological variations in the thickness of
the vaginal epithelium were coincident with
the reproductive status, taking place a great
desquamation during the estrous. Mayor et al.
11
reported that in Atherurus africanus the vaginal
content and the epithelium vary in accordance
with the reproductive status. In diestrous, females
of this species showed no cornification and less
than 5 cellular layers. During the follicular phase,
there was an increase of the stratification and
cornification of the epithelium and the vaginal
content presented abundant eosinophilic cells.
Similar observations were realized in the wild
black agouti (Dasyprocta fuliginosa)12.
The vaginal epithelium is sensitive to the
effect of the sexual steroids, mainly estrogens,
and presents predictable changes throughout
the estrous cycle in response to the changes in
plasma concentrations of ovarian hormones.
The increase in the estrogen levels determines
the keratinization of the vaginal epithelium. This
keratinization of the vaginal epithelium has been
used as an indicator of the biological activity of
circulating sexual steroids in many mammalian
species and to determine the appropriate time for
mating. Estrogens, both natural and exogenous,
possess a direct effect on the vaginal epithelium22.
When an increase of the circulating estrogen
levels takes place, the growth of the number of
cellular strata and cornification of the uppermost
superficial layers are stimulated. Hence, when
the estrogen levels decrease, an extensive
desquamation of the vaginal epithelium occurs.
These variations determine different cellular
scenes during the estrous cycle.
CONCLUSION
Results of the present study demonstrate
that the estrous cycle of the M. c. bonariensis
is characterized by its long duration. The
composition of the vaginal smears showed a
sequence that is very similar to other rodents.
Differences in the epithelial thickness observed
between the different vaginal regions suggest that
not all the vaginal areas respond in a similar way
to the ovarian hormones. The lesser epithelial
thickness in the metestrous, compared to that in
the diestrous, may be interpreted as that during
this last phase the follicular growth would be
taking place. This could suggest that an increase
of plasma estrogens is occurring in the female.
The information presented in this
study show the necessity of more research
studies, suggesting that more samples during the
intermediate stages of the diestrous and a correlation
between macroscopical observations and ovarian
status could allow a better comprehension of the
changes that occur during the estrous cycle in coypu.
Ethics: Females were sacrificed according to the
methods established by the Animal Welfare Act
of the Facultad de Ciencias Veterinarias de la
Universidad Nacional del Centro de la Provincia
de Buenos Aires (2002)3.
Conflict of interest: The authors declare that
they have no conflict of interest.
1. Addo, P.G.; Awumbila, B.; Awotwi, E.; Ankrah, N.A.
Comparative characterization of the oestrous cycles
of the grasscutter (Thryonomys swinderianus) and the
guinea pig (Cavia porcellus) by the hystricomorph vaginal
membrane perforation phenomenon. Livestock Research
for Rural Development 2007; 19(5). En: http://www.lrrd.
org/lrrd19/5/addo19063.htm
2. Almeida, C.; Pinheiro, P.F.; Segatelli, T.M.; Martinez, M.;
Padovani, C.; Martinez, F. Estrous cycle, anatomy and
histology of the uterine tube of the mongolian gerbil
(Meriones unguiculatus). Revista Chilena de Anatomía
2001; 19: 191-196.
3. Animal Welfare Act. Facultad de Ciencias Veterinarias,
UNCPBA. En: http//:www.vet.unicen.edu.ar. 2002.
4. Courtalon, P.; Bó, R.F.; Spina, F.; Jiménez, N.; Cantil, L.;
Fernández, R.; Porini, G. Reproductive ecology of coypu
(Myocastor coypus) Molina 1782 in the Middle Delta of
the Paraná River, Argentina. Brazilian Journal of Biology
2015; 75(1): 30-38.
5. Felipe, A.; Callejas, S.S.; Cabodevila, J.
Anatomicohistological characteristics of female genital
tubular organs of the South American nutria (Myocastor
coypus). Anatomia, Histologia, Embryologia 1998; 27:
245-250.
6. Felipe, A.; Callejas, S.S.; Cabodevila, J. Anatomohistological
Characteristics of the ovary of the south american nutria
(Myocastor coypus). Anatomia, Histologia, Embryologia
1999; 28: 89-95.
7. Felipe, A.; Callejas, S.S.; Cabodevila, J. Characterization
of the estrous cycle of Myocastor coypus (coypu) by
means of exfoliative colpocytology. Journal of Neotropical
Mammalogy 2001; 8: 129-137.
8. Grégoire, A.; Allard, A.; Huamán, E. et al. Control of
the estrous cycle in guinea-pig (Cavia porcellus).
Theriogenology 2012; 1: 842-847.
9. Mahoney, M.; Rossi, B.; Hagenauer, M.; Lee, T.
Characterization of the estrous cycle in Octodon degus.
Biology of Reproduction 2011; 84: 664–671.
10. Malekani, M.; Westlin, L.; Paulus, J.; Potgieter, H. Oestrous
occurrence in captive female Cricetomys gambianus
(Rodentia: Cricetidae). Journal of Zoology 2002; 257: 295-
301.
11. Mayor, P.; López-Béjar, M.; Jori. F.; et al. Reproductive
functional anatomy and oestrous cycle pattern of the
female brush-tailed porcupine (Atherurus africanusz, Gray
1842) from Gabon. Animal Reproduction Science 2003;
77: 247-259.
12. Mayor, P.; Bodmer, R.; Lopez-Bejar, M. Functional
anatomy of the female genital organs of the wild black
agouti (Dasyprocta fuliginosa) female in the Peruvian
Amazon. Animal Reproduction Science 2011; 123: 249-
257.
13. Mori, E.; Menchetti, M.; Lucherini, M.; Lovari, S. Timing of
reproduction and paternal cares in the crested porcupine.
Mammalian Biology 2016; 81(4): 345-349.
14. . Ojasti, J. Manejo de fauna silvestre neotropical. SIMAB
Series Nro. 5, Smithsonian Institution/MAB Program,
Washington DC. 2000.
15. . Suckow, M.; Stevens, K.; Wilson, R. (eds.) The laboratory
rabbit, guinea pig, hamster, and other rodents. American
College of Laboratory Animal Medicine. Academic Press,
Nueva York. 2012.
16. Torres Rodrigues, J.; Vieira Ferro, E. Morphological
changes in the vaginal epithelium during the oestrous
cycle of Calomys callosus (Rodentia, Cricetidae). Revista
Brasilera de Biologia 1998; 58: 527-539.
17. Touma, C.; Palme, R.; Sachser, N. Different types of
oestrous cycle in two closely related South American
rodents (Cavia aperea and Galea musteloides) with
different social and mating systems. Reproduction 2001;
121: 791-801.
18. Valladares, P.; Spotorno, A; Cortes, A.; Zuleta, C. Chinchilla
chinchilla (Rodentia: Chinchillidae). Mammalian Species
2018; 50(960): 51–58.
19. Verharen, J.; Kentrop, J.; Vanderschuren, R.; Adan, R.
Reinforcement learning across the rat estrous cycle.
Psychoneuroendocrinology 2019; 100: 27–31.
20. Weir, B.J. Some observations on reproduction in the
female agouti, Dasyprocta agouti. Journal of Reproduction
and Fertility 1971a; 24: 203-211.
21. Weir, B.J. Some observations on reproduction in the
female green accouchi, Myoprocta pratti. Journal of
Reproduction and Fertility 1971b; 24: 193-201.
22. Wessels, J.; Felker, A.; Dupont, H.; Kaushic, C. The
relationship between sex hormones, the vaginal
microbiome and immunity in HIV-1 susceptibility
in women. Disease Models & Mechanisms. 2018;
11(9):dmm035147.
23. Society of Critical Care Medicine, American Association for
Respiratory Care, American Society of Anesthesiologists,
Anesthesia Patient Safety Foundation, American Association of Critical-Care Nurses, and American College of Zipser, B.; Schleking, A.; Kaiser, S.; Sachser, N. Effects of
domestication on biobehavioural profiles: a comparison
of domestic guinea pigs and wild cavies from early to
late adolescence. Frontiers in Zoology 2014; 11(30). En:
https://frontiersinzoology.biomedcentral.com/track/
pdf/10.1186/1742-9994-11-30.pdf