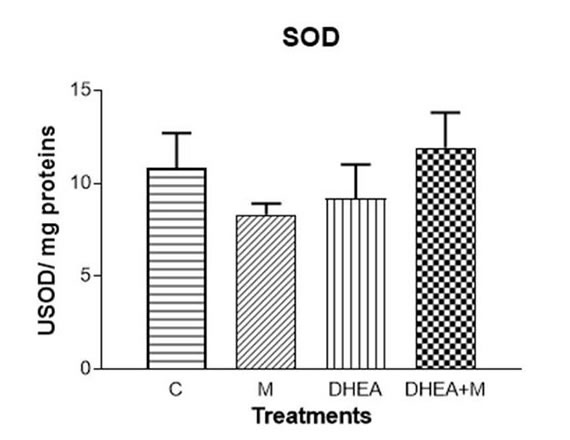
Figure 1A. Redox status in uterine homogenates. SOD activity.
ARTÍCULO DE INVESTIGACIÓN
Antioxidant effect of metformin in a murine model of hyperandrogenized pregnancy
Luchetti, C.G. 1, 3; Motta, A.B.2, 3; Lombardo, D.M.1
1Universidad de Buenos Aires, Facultad de Ciencias Veterinarias, Instituto de Investigación y Tecnología en Reproducción Animal (INITRA), Cátedra de Histología y Embriología.
2Universidad de Buenos Aires, Facultad de Medicina, Laboratorio de Fisiopatología Ovárica, Departamento de Farmacología, Centro de Estudios Farmacológicos y Botánicos (CEFYBO), Buenos Aires, Argentina.
3Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET).
Recibido: 20/12/2018
Aceptado: 27/05/2019
Correspondencia e-mail: Daniel M. Lombardo dlombard@fvet.uba.ar
Summary
Hyperandrogenization is one of the main clinical features of the polycystic ovarian syndrome (PCOS). Metformin (M) is a non-hormonal treatment used in PCOS even during pregnancy. The objective was to study the effects of M on the redox balance and the nitric oxide (NO) system in a murine model of early hyperandrogenized pregnancy. Early pregnant Balb/ c mice hyperandrogenized by dehydroepiandrosterone (DHEA) and treated orally with M were used. Redox and NO system parameters were determined at the implantation sites. DHEA increased oxidative stress: lipid peroxidation (TBA-RS, p <0.01) and glutathione (Tietze; p <0.01). With DHEA + M, TBA-RS was similar to the control and glutathione was similar to the DHEA group. The enzymes superoxide dismutase and catalase did not show differences. DHEA caused an increase in the expression of inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS) (immunohistochemistry) and in NO (Griess; p <0.001 in all cases). With DHEA+M they were similar to the control. We conclude that M avoids the effects of DHEA on oxidative stress and the NO system in the implantation sites of hyperandrogenized early pregnant Balb/c mice.
Key words: DHEA; Oxidative stress; NO; Polycystic ovary syndrome; Implantation sites.
Efecto antioxidante de metformina en un modelo murino de preñez hiperandrogenizada
Resumen
La hiperandrogenización es una de las características clínicas principales del síndrome de ovario poliquístico (SOP). La metformina (M) es un tratamiento no hormonal utilizado en el SOP incluso durante el embarazo. El objetivo fue estudiar los efectos de M sobre el balance redox y el sistema óxido nítrico (ON) en un modelo murino de preñez temprana hiperandrogenizada. Se utilizaron ratones Balb/ c hiperandrogenizados por dehidroepiandrosterona (DHEA) durante la preñez temprana y tratados oralmente con M. Se determinaron parámetros redox y del sistema ON en los sitios de implantación. La DHEA incrementó el estrés oxidativo: peroxidación lipídica (TBA-RS; p <0.01) y glutatión (Tietze; p <0.01). Con DHEA+M, TBA-RS fue similar al control y el glutatión fue similar al grupo DHEA. Las enzimas superóxido dismutasa y catalasa no mostraron diferencias. La DHEA provocó un aumento en la expresión de óxido nítrico sintetasa inducible (ONSi) y óxido nítrico sintetasa endotelial (ONSe) (inmunohistoquímica) y en ON (Griess; p <0,001 en todos los casos). Con DHEA+M fueron similares al control. Concluimos que M evita los efectos de DHEA sobre el estrés oxidativo y el sistema ON en los sitios de implantación de ratones Balb/ c durante la preñez temprana hiperandrogenizada.
Palabras clave: DHEA; Estrés oxidativo; ON; Síndrome del ovario poliquístico; Sitios de implantación.
Introduction
An oxidative stress status is the product of the imbalance between the antioxidant defenses on the one side and the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) on the other side. The direct damaging result of oxidative stress is the oxidation of lipids, proteins and DNA. ROS are formed from the incomplete reduction of O2. RNS are oxidative reaction products of NO; one of them, peroxynitrite, is highly reactive at physiological pH values. Oxidative stress may decline nitric oxide’s bioavailability because they are very ineffective sources for NO. Excessive, uncontrolled, and unmanaged oxidative stress can lead to diseases27. NO is an essential metabolite involved in vascular function and in numerous physiological processes that maintain homeostasis. However, when the vascular system is diseased, and defense mechanisms are activated, the mediators of inflammation induce inducible nitric oxide synthase (iNOS), which produce large quantities of NO in most of the cells of the vessel wall2, 28. In pregnancy, NO plays important roles in implantation, decidualization, vasodilatation of decidual, placental and uterine vessels and myometrial relaxation41. It has been reported that NO participates in the vascular invasion by the trophoblast, and it may play a role in maintaining uterine quiescence during pregnancy6, 16. However, NO could have a dual action (protective or pro-oxidant) during the corpus luteum development30. It also has a role in complicated pregnancies with a pro-oxidant and a pro-inflammatory status, associated with preeclampsia, intrauterine growth restriction, pregestational diabetes and miscarriage10, 33. Besides, it was found that endothelial nitric oxide synthase (eNOS) gene influences the risk of pre-eclampsia and the recurrence of negative pregnancy events11. The production of NO is due to three isoforms of the NO synthase: the Ca-dependent constitutively expressed eNOS and neuronal NOS (nNOS), and the non-responsive to calcium iNOS. Although NO synthases iNOS and eNOS have been identified in rodent uterus via western blot analysis and immunohistochemistry during implantation and late gestation1, 35, very few localization and quantification studies of NOS and NO during early pregnancy have been performed. Dehydroepiandrosterone (DHEA) is the most abundant androgen found in women with polycystic ovary syndrome (PCOS)48. This hormone is involved in immune homeostasis and is increased in normal pregnancy contributing to the development of gestation44. However, abnormally increased DHEA levels lead to an imbalance in ovarian function and to detrimental effects on the endometrial function that result in low implantation rates and miscarriage8, 34. Simoncini et al. 2003 have reported that DHEA induces NO synthesis by a direct effect on eNOS41. We have previously developed a murine model which reflects endocrine and immune aspects of women with PCOS during early pregnancy by hyperandrogenization with DHEA31, 38. The biguanide insulin-sensitizing drug M is a non-hormonal treatment for a wide variety of diseases such as type II diabetes mellitus9, PCOS15 and some types of cancer19, 20, 37, 47. These conditions are accomplished with a decreased antioxidant capacity that could contribute to the known increased risk of cardiovascular diseases like atherosclerosis or hypertension in patients with diabetes and PCOS12, 40. This drug has shown to reduce oxidative stress in numerous systems. Apparently, it exerts its antioxidant effects directly scavenging ROS or indirectly modulating the intracellular ROS production3, 24. In women with PCOS, it has been seen that M treatment decreases androgen levels, improves the frequency of menstrual cycle and ovulation24, 31 and prevents abortions in early pregnant women with PCOS13, 18, 46. There is an increasing number of women with PCOS that became pregnant after M treatment and that maintain treatment during pregnancy. However, some aspects of the mechanism involved are not completely known. The aim of this work was to study the effects of M on the redox balance and the nitric oxide system in a murine model of DHEA- hyperandrogenized early pregnancy.
Materials and methods
Animals
The animal model was previously described43. Briefly, 8 to 12- week- old virgin female BALB/c mice were paired with 8 to 12- week- old BALB/c males. The day of appearance of a coital plug was taken as day 0 of pregnancy. Implantation occurs on the morning of the 5th day, therefore, on 6th and 7th days (post-implantation) animals were divided in 4 groups: control (supplied only with vehicles), M (treated orally by cannula with 240 mg/kg of metformin in 0.1 ml of water), DHEA (injected s.c. with 60 mg/kg of dehydroepiandrosterone in 0.1 ml of sesame oil) and DHEA+M (treated under the same conditions with DHEA and M). On day 8 of pregnancy, animals were euthanized by cervical dislocation. After embryos were removed, uterine tissues from 14 animals per group were divided as follows: 7 uteri of each group were immediately homogenized in buffer Tris-Base 20 mM, pH= 7,6 to determine redox parameters (superoxide dismutase, catalase, lipid peroxidation, glutathione and nitrite concentration). The remaining 7 uteri were immediately fixed in 4 % (w/v) paraformaldehyde to carry out the immunohistochemical determination of eNOS and iNOS.
To test any long-term adverse effects of the treatments 3 additional groups (10 animals each) including control, M and DHEA+M mice were allowed to proceed to parturition. These animals had a normal pregnancy with a normal number and morphology of pups. The DHEA group was not included because the pregnancy with this treatment did not continue in the majority of these females.
The mice were housed under controlled temperature (22 ºC) and illumination (14 h light, 10 h darkness; lights on at 05:00) and were allowed to free access to Purina rat chow and water. All procedures involving animals were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Experimental procedures
Redox related parameters. Uterine tissues without embryos were homogenized in Buffer Tris-Base 20 mM, pH=7,6 with a Teflon-glass homogenizer in ice. The suspension was centrifuged at 4500 x g for 10 min at 4C. The supernatant was saved and stored at –20º C for the redox determinations described ahead and for the protein content determination by Bradford method4. Catalase was measured by the method described by Maehly and Chance in 195423. Results were expressed as catalase content in pmol catalase/ mg protein. Superoxide dismutase (SOD) was determined by the method described by Misra and Fridovich in 197226. Results were expressed as USOD/ mg protein. Lipid peroxidation was detected by the method of TBA reactive species (TBA-RS), described by Buege and Aust in 19785. Results were expressed as nmol MDA/ mg protein. Glutathione was quantified by the method described by Tietze in 196945. Results were expressed as mol GSH/mg protein. Nitric oxide was valued by nitrites (NO2) concentration by Griess reaction14. Results were expressed as nmol NO2/ mg protein.
Immunohistochemical localization of iNOS and eNOS. Uterine slides were stained with the immunoperoxidase staining kit CSA/HRP (Dako). Briefly, uterine tissues fixed in paraformaldehyde 4 % were embedded in paraffin wax, consecutively cut (6 m/section) and placed on glass slides covered by xylane (Biobond; British Biocell International, Cardiff). Only sections that passed through the center of the implantation sites were selected. Then, tissue sections were dewaxed, rehydrated through a series of graded alcohols and washed in PBS. Endogenous peroxidase activity was blocked with 0.1 % (v/v) hydrogen peroxide for 50 min and non-specific binding sites were blocked by treating tissues with TNB blocking reagent (NEN Life Science Products, Boston, Ma, USA). Then, sections were incubated overnight at 4 ºC with the primary rabbit polyclonal anti-human eNOS, and iNOS (Cayman, USA) antibodies respectively diluted 1:200 in blocking buffer. Control sections were made without the primary antibody. Sections were then incubated for 30 min with biotinylated secondary anti-rabbit antibody, washed in PBS and treated with streptavidin-biotin complex for 30 min. The reaction was visualized by diaminobenzidine (DAB staining Kit, Dako) and sections were counterstained with hematoxylin and covered with DPX (Sigma). A morphometric study was carried out including the quantification of the immune mark through a digital imaging system of analysis (Image-Pro Plus version 4.1 software, Media Cybernetics, Silver Spring, Md) in which marked area per measured area was determined. Results were expressed as the percentage of marked area/ measured area.
Statistical analysis. Statistical analyses were carried out using the Instat program (Graph Pad Software, San Diego, CA, USA). One way ANOVA test (Tukey post-test Multiple Comparison that compares all the pairs of columns) was used for SOD, catalase, lipid peroxidation and NO. Chi-square test (Fisher´s exact test for differences between columns) was used for analyzing the proportions of marked/ total area. A P value < 0.05 was considered significant. Results were presented as mean values ± SEM.
Results
Redox status
The antioxidant enzymes SOD and catalase showed no differences in any of the treatments (Fig. 1 A and B respectively). Lipid peroxidation increased with DHEA (p<0.01). This increase was avoided by DHEA+M (Fig. 1 C). Glutathione increased with DHEA (p<0.01) and also with DHEA+M (p<0.01, Fig. 1 D). The concentration of NO significantly increased with DHEA (p<0.001), whereas DHEA+M treatment prevented this effect (Fig. 1 E)

Figure 1A. Redox status in uterine homogenates. SOD activity.
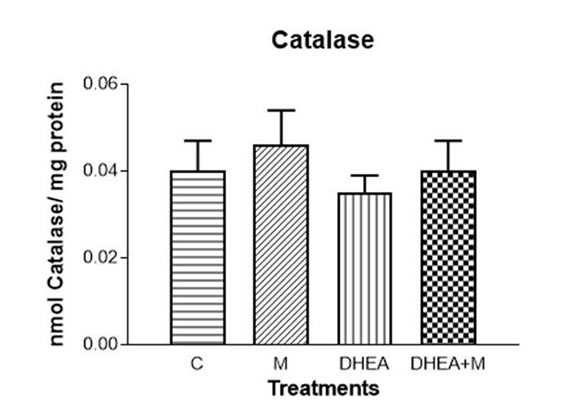
Figure 1B. Redox status in uterine homogenates. Catalase activity.
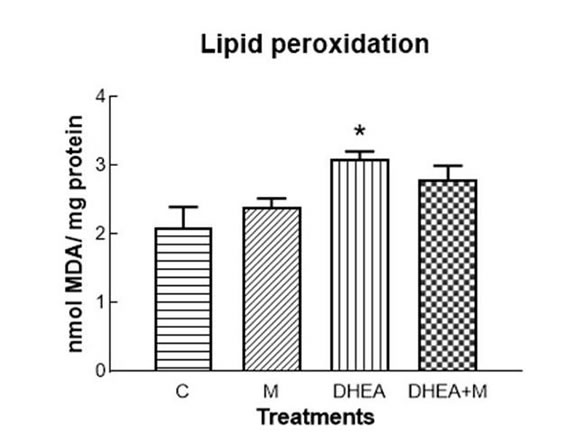
Figure 1C. Redox status in uterine homogenates. Lipid peroxidation (*= p<0.01).
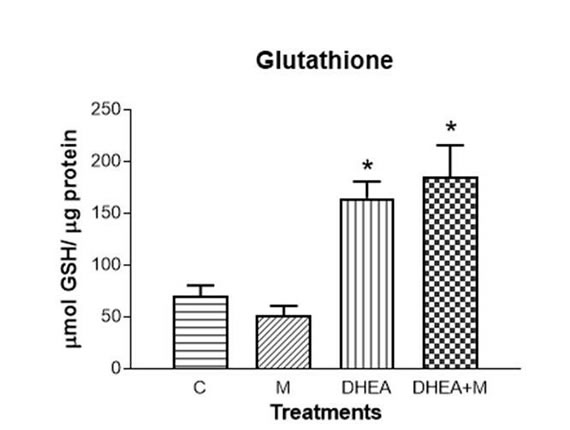
Figure 1D. Redox status in uterine homogenates. Glutathione (*=p<0.01)

Figure 1E. Redox status in uterine homogenates. NO concentration (*=p<0.001).
Expression of iNOS and eNOS in implantation sites
iNOS immunoreactivity was present in trophoblastic cells (tr). This was seen in implantation sites from control and DHEA+M groups (Fig. 2 A and C: tr). However, in DHEA treated mice this enzyme showed an increased expression on the decidual matrix when compared to control (Fig. 2 B: mesometrial decidua, md, and antimesometrial decidua, amd). Image-Pro Plus quantified stained/total area revealed a significantly increased expression of iNOS in DHEA treated mice, avoided with DHEA+M treatment (Fig. 2 D).
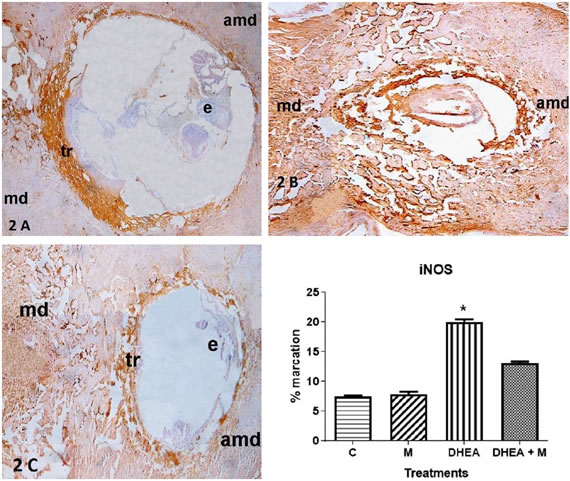
Figure 2. 2A. Expression of iNOS in implantation sites. iNOS immunohistochemistry of Control group. 2B. Expression of iNOS in implantation sites. iNOS immunohistochemistry of DHEA group. 2C. Expression of iNOS in implantation sites. iNOS immunohistochemistry of DHEA+M. e: embryo, tr: trophoblastic cells, md: mesometrial decidua, amd: antimesometrial decidua. 2D. Expression of iNOS in implantation sites. Quantification of iNOS expression (*=p<0.01).
eNOs was also present in trophoblastic cells (tr) in implantation sites from control and DHEA+M treated animals (Fig. 3A and C: tr). In DHEA treated mice, eNOS showed an increased expression on the decidual matrix (Fig. 3 B: mesometrial decidua, md, and antimesometrial decidua, amd). Quantification of the stained/ total area showed a significantly increased expression of eNOS with DHEA, completely avoided with DHEA+M (Fig. 3D).
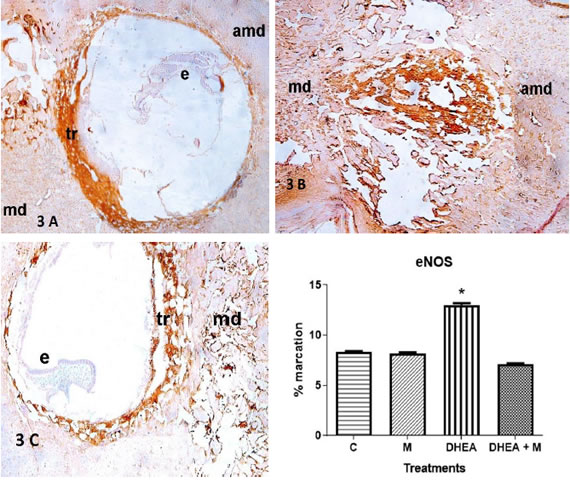
Figure 3. 3A. Expression of eNOS in implantation sites. eNOS immunohistochemistry of Control group. 3B. Expression of eNOS in implantation sites. eNOS immunohistochemistry of DHEA group. Figure 3C. Expression of eNOS in implantation sites. eNOS immunohistochemistry of DHEA+M. e: embryo, tr: trophoblastic cells, md: mesometrial decidua, amd: antimesometrial decidua. Figure 3D. Expression of eNOS in implantation sites. Quantification of eNOS expression (*=p<0.01).
The expression of these NOS isoforms in tissues obtained from M treated mice showed an appearance similar to controls (data not shown).
Discussion
DHEA is the most abundant androgen in PCOS48. It induces a pro-inflammatory environment and triggers the synthesis of NO21, 22, 29. Besides, it induces the loss of luteal function and embryo resorption5, 43. The present model resembles the endocrine and immune situation of early pregnant women with PCOS treated with M22, 43. We studied the antioxidant effects of M in this model with a connection to the NO system. We propose that oxidative stress has an important role in the cascade of events triggered by DHEA hyperandrogenization that leads to embryo resorption. This is shown in the increased levels of lipid peroxidation, NO, iNOS and eNOS in DHEA-hyperandrogenized females. We propose that DHEA has a direct effect on the NO system, increasing the expression of NOS and the production of NO, in agreement with a previous report about this direct effect41. At this point, there probably exists a down-regulation exerted by the overproduced NO on the activity of this enzyme, reflected in the decrease in NOS activity seen in a previous work43. The regulation of NOS activity but not of its expression has been seen in other models17, 36 and could be due to the acute treatment used here. In this work, unlike previous studies 33, 35, we see that iNOS and eNOS are located in the trophoblastic cells, being of embryonic origin. This localization is possibly due to the participation of NO in the vascular invasion by the trophoblast6, 16. Besides, we found that DHEA hyperandrogenization increases the expression of iNOS and eNOS in these sites, and their product, NO. As a consequence of the NO increase, there is an oxidative stress status in implantation sites of these mice. This is reflected here in the high levels of lipid peroxidation.
The treatment with M prevents oxidative stress and the increase of NOS expression and NO production. In previous reports, we have seen protective effects of M in the ovaries and uterus of DHEA-hyperandrogenized mice7, 22, 43. M acts here as an antioxidant, avoiding the increase in lipid peroxidation triggered by DHEA. Glutathione is an instantaneous antioxidant defense, and its increased levels could be an attempt to increase the antioxidant defense. The antioxidant properties of M have been shown in a variety of models3, 24.
This work is a new step in the knowledge of the mechanism of action of M during early hyperandrogenized pregnancy. This new contribution adds to the findings we had previously shown using the same experimental model where M prevents endocrine and immune alterations induced by DHEA. All these factors cooperate in a physiopathological path whose final result is the continuity of pregnancy or embryo resorption: glucose, serum estrogen and progesterone, progesterone-induced blocking factor (PIBF) and cyclooxygenase 2 (COX2) at implantation sites, cytokine production and oxidative stress in the ovaries and uterine NOS activity are altered22, 43 and the final result is that DHEA increases embryo resorption (88 +/- 1 %), whereas DHEA+M avoids this effect (43 +/- 3% v. 35 +/- 5% in controls)43.
Conclusion
DHEA induces, and M prevents, a detrimental increase of oxidative stress and NO in implantation sites during early pregnancy in mice. The treatment with M seems to be a good option for maintaining pregnancy in patients with PCOS. Regulation of nitric oxide by M probably favors the normal vascular function in these patients. It could contribute to decreasing the risk of cardiovascular diseases as atherosclerosis or hypertension seen in patients with diabetes and PCOS. Besides, M is a non-hormonal treatment and did not show to be teratogenic transforming it in a good choice for PCOS patients. There remain some aspects to be studied, including longtime effects of this treatment in pregnancy.
Acknowledgements
Authors would like to express their gratitude to Dr. Dante Paz for his great human and technical support, and for his kind advice and suggestions.
1. Ali, M; Buhimschi, I; Chwalisz, K; Garfield, RE. Changes in expression of the nitric oxide synthase isoforms in rat uterus and cervix during pregnancy and parturition. Mol Hum Reprod 1997; 3(11):995-1003.
2. Andrew, PJ; Mayer, B. Enzymatic function of nitric oxide synthases. Cardiovasc Res 1999; 43 (suppl 3):521-31.
3. Bonnefont-Rousselot, D; Raji, B; Walrand, S; Gardès-Albert, M; Jore, D; Legrand, A et al. An intracellular modulation of free radical production could contribute to the beneficial effects of metformin towards oxidative stress. Metabolism 2003; 52 (suppl 5):586-9.
4. Bradford, MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248-254.
5. Buege, JA; Aust, SD. Microsomal lipid peroxidation. Methods Enzymol 1978; 52:302-10
6. Chwalisz, K and Garfield, RE. Role of nitric oxide in implantation and menstruation. Hum Reprod 2000; 15:96–111.
7. Elia, E; Sander, V; Luchetti, CG; Solano, ME; Di Girolamo, G; Gonzalez, C et al. The mechanisms involved in the action of metformin in regulating ovarian function in hyperandrogenized mice. Mol Hum Reprod 2006; 12:475-481.
8. Elia, E; Vighi, S; Lombardi, E; Motta, AB. Detrimental effects of hyperandrogenism on uterine functions. Int Immunopharmacol 2008; 8 (suppl 13-14):1827-34.
9. Eriksson, A; Attvall, S; Bonnier, M; Eriksson, JW; Rosander, B; Karlsson, FA. Short-term effects of metformin in type 2 diabetes. Diabetes Obes Metab 2007; 9 (suppl 4):483-9.
10. Estevez, A; Tognetti, T; Rearte, B; Sander, V; Motta, AB. Interleukin-1beta in the functional and structural luteolysis. Relationship with the nitric oxide system. Prostaglandins Leukot Essent Fatty Acids 2002; 67 (suppl 6):411-7.
11. Fatini, C; Sticchi, E; Gensini, F; Genuardi, M; Tondi, F; Gensini, GF et al. Endothelial nitric oxide synthase gene influences the risk of pre-eclampsia, the recurrence of negative pregnancy events, and the maternal-fetal flow. J Hypertens 2006; 24 (suppl 9):1823-9.
12. Fenkci, V; Fenkci, S; Yilmazer, M; Sertesser, M. Decreased total antioxidant status and increased oxidative stress in women with Polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil Steril 2003; 123-127.
13. Glueck, CJ; Wang, P; Goldenberg, N; Sieve-Smith, L. Pregnancy outcomes among women with polycystic ovary syndrome treated with metformin. Hum Reprod 2002; 17 (suppl 11):2858-2864.
14. Griess, JP. On a new series of bodies in which nitrogen is substituted for hydrogen. Philos Trans R Soc (London) 1864; 154:667–731.
15. Harborne, L; Fleming, R; Lyall, H; Norman, J; Sattar, N. Descriptive review of the evidence for the use of metformin in polycystic ovary syndrome. Lancet 2003; 361: 1894–1901.
16. Harris, LK; McCormick, J; Cartwright, JE; Whitley, GS; Dash, PR. S-nitrosylation of proteins at the leading edge of migrating trophoblasts by inducible nitric oxide synthase promotes trophoblast invasion. Exp Cell Res 2008; 314 (suppl 8):1765-76.
17. Jawerbaum, A; Gonzalez, E. The role of alterations in arachidonic acid metabolism and nitric oxide homeostasis in rat models of diabetes during early pregnancy. Curr Pharm Des 2005; 11 (suppl 10):1327-42.
18. Khattab, S; Mohsen, IA; Foutouh, IA; Ramadan, A; Moaz, M; Al-Inany, H. Metformin reduces abortion in pregnant women with polycystic ovary syndrome. Gynecol Endocrinol 2006; 22:680-684.
19. Kruk, PJ. Beneficial effect of additional treatment with widely available anticancer agents in advanced small lung cell carcinoma: A case report. Mol Clin Oncol 2018; 9(6):647-650.
20. Liu, X; Romero, IL; Litchfield, LM; Lengyel, E; Locasale, JW. Metformin Targets Central Carbon Metabolism and Reveals Mitochondrial Requirements in Human Cancers. Cell Metab 2016; 24(5):728-739.
21. Luchetti, CG; Solano, ME; Sander, V; Arcos, ML; Gonzalez, C; Di Girolamo, G et al. Effects of dehydroepiandrosterone on ovarian cystogenesis and immune function. J Reprod Immunol 2004; 64 (suppl 1-2):59-74
22. Luchetti, CG; Mikó, E; Szekeres-Bartho, J; Paz, DA; Motta, AB. Dehydroepiandrosterone and metformin modulate progesterone-induced blocking factor (PIBF), cyclooxygenase 2 (COX2) and cytokines in early pregnant mice. J Steroid Biochem Mol Biol 2008; 111 (suppl 3-5):200-7.
23. Maehly, AC; Chance, B. The assay of catalases and peroxidases. Methods Biochem Anal 1954; 1:357-424.
24. Mahrouf, M; Ouslimani, N: Peynet, J; Djelidi, R; Couturier, M; Therond, P et al. Metformin reduces angiotensin-mediated intracellular production of reactive oxygen species in endothelial cells through the inhibition of protein kinase C. Biochem Pharmacol 2006; 72 (suppl 2):176-83.
25. Mansfield, R; Galea, R; Brincat, M; Hole, D; Mason, H. Metformin has direct effects on human ovarian steroidogenesis. Fertil Steril 2003; 79 (suppl 4):956-962.
26. Misra, HP; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972; 247 (suppl 10):3170-5.
27. Modun, D; Giustarini, D; Tsikas, D. Nitric oxide-related oxidative stress and redox status in health and disease. Oxid Med Cell Longev 2014; 2014:129651.
28. Moncada, S; Palmer, RM; Higgs, EA. The discovery of nitric oxide as the endogenous nitrovasodilator. Hypertension 1988; 12 (suppl 4):365-72.
29. Motta, AB; Estevez, A; de Gimeno, MF. The involvement of nitric oxide in corpus luteum regression in the rat: feedback mechanism between prostaglandin F (2alpha) and nitric oxide. Mol Hum Reprod 1999; 5 (suppl 11), 1011-6.
30. Motta, AB; Estevez, A; Tognetti, T; Gimeno, MA, Franchi, AM. Dual effects of nitric oxide in functional and regressing rat corpus luteum. Mol Hum Reprod 2001; 7 (suppl 1):43-7.
31. Motta, AB. Dehydroepiandrosterone to induce murine models for the study of polycystic ovary syndrome. J Steroid Biochem Mol Biol 2010;119(3-5):105-11.
32. Nestler, JE; Jakubowicz, DJ. Decreases in Ovarian Cytochrome P450c17 alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med 1996; 335 (suppl 9):617-623.
33. Ogando, DG; Paz, D; Cella, M; Franchi, AM. The fundamental role of increased production of nitric oxide in lipopolysaccharide-induced embryonic resorption in mice. Reproduction 2003; 125: 95–110.
34. Okon, MA; Laird, SM; Tuckerman, EM; Li, TC. Serum androgen levels in women who have recurrent miscarriages and their correlation with markers of endometrial function. Fertil Steril 1998; 69 (suppl 4):682-90.
35. Purcell, TL; Given, R; Chwalisz, K; Garfield, RE. Nitric oxide synthase distribution during implantation in the mouse. Mol Hum Reprod 1999; 5 (suppl 5):467–475.
36. Rengasamy, A; Johns, RA. Inhibition of nitric oxide synthase by a superoxide generating system. J Pharmacol Exp Ther 1993; 267 (suppl 3):1024-7.
37. Ryabaya, O; Prokofieva, A; Akasov, R; Khochenkov, D; Emelyanova, M; Burov, S et al. Metformin increases antitumor activity of MEK inhibitor binimetinib in 2D and 3D models of human metastatic melanoma cells. Biomed Pharmacother 2019; 109:2548-2560.
38. Sander, V; Solano, ME; Gutierrez, M; Luchetti, CG; Rearte, MB; Gonzalez, C et al. The influence of Dehydroepiandrosterone on Early Pregnancy in Mice. Neuroimmunomodulat 2005; 12:285-292.
39. Sander, VA; Piehl, L; Facorro, GB; Rubín de Celis, E; Motta, AB. Regulation of functional and regressing stages of corpus luteum development in mice. Role of reactive oxygen species. Reprod Fertil Dev 2008; 20 (suppl 7):760-9.
40. Sena, CM; Nunes, E; Louro, T; Proença, T; Seiça, RM. Endothelial dysfunction in type 2 diabetes: effect of antioxidants. Rev Port Cardiol 2007; 26 (suppl 6):609-19.
41. Simoncini, T; Mannella, P; Fornari, L; Varone, G; Caruso, A; Genazzani, AR. Dehydroepiandrosterone modulates endothelial nitric oxide synthesis via direct genomic and nongenomic mechanisms. Endocrinol 2003; 144 (suppl 8):3449-55.
42. Sladek, SM; Magness, RR; Kirk, PC. Nitric oxide and pregnancy. Am J Physio 1997; 272:441-463.
43. Solano, ME; Elia, E; Luchetti, CG; Sander, V; Di Girolamo, G; Gonzalez, C et al. Metformin prevents embryonic resorption induced by hyperandrogenisation with dehydroepiandrosterone in mice. Reprod Fertil Dev 2006; 18 (suppl 5):533-44.
44. Tagawa, N; Hidaka, Y; Takano, T; Shimaoka, Y; Kobayashi, Y; Amino, N. Serum concentrations of dehydroepiandrosterone and dehydroepiandrosterone sulfate and their relation to cytokine production during and after normal pregnancy. Clin Chim Acta 2004; 340 (suppl 1-2):187-93.
45. Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 1969; 27 (suppl 3):502-22.
46. Vandermolen, DT; Ratts, VS; Evans, WS; Stovall, DW; Kauma, SW; Nestler, JE. Metformin increases the ovulatory rate and pregnancy rate from clomiphene citrate in patients with polycystic ovary syndrome who are resistant to clomiphene citrate alone. Fertil Steril 2001; 75:310–315.
47. Xin, M; Wang, Y; Ren, Q; Guo, Y. Formononetin and metformin act synergistically to inhibit growth of MCF-7 breast cancer cells in vitro. Biomed Pharmacother 2019; 109:2084-2089.
48. Yoshino, K; Takahashi, K; Eda, Y; Okada, S; Kitao, M. Endocrinological environment with regard to the number of microcysts in patients with polycystic ovary syndrome. Hum Reprod 1992; 7(suppl 9):1201-4..