ARTÍCULO DE INVESTIGACIÓN
Experimental model to assess the Tritrichomonas foetus vaccine- induced effects
Soto, P.; Echevarría, H.M.; Doumecq, M.L.; Cacciato, C.S.; Rodriguez, E.; Monteavaro, C.E.
NACT SAMP, CIVETAN-CONICET. School of Veterinary Sciences, National University of the Center of the Buenos Aires Province. Campus Universitario, Arroyo seco s/n, Tandil, Buenos Aires, Argentina.
Recibido: 25/06/2018
Aceptado: 19/12/2018
Correspondencia e-mail: Pedro Soto psoto@vet.unicen.edu.ar
Summary
The purpose of this work was to present an experimental murine model as an alternative to the bovine one, to evaluate the efficacy of experimental vaccines against Tritrichomonas foetus. The vaccines employed consisted of inactivated protozoa with different formulations: (A) with oil adjuvant, (B) aqueous vaccine, (C) aqueous vaccine with dimethyl sulphoside. Seventy female BALB/c mice were used and distributed in 7 experimental groups; each group received 2 doses of vaccine, two groups received a vaginal booster at the time of the second dose. Fifteen days after the second dose, all animals were challenged by vaginal instillation with T. fetus. Blood samples and vaginal mucus were taken until day 30 post infection. The immune response was evaluated by ELISA and the vaginal infection assessed by vaginal mucus culture in the TYM medium. The greatest immune response was related to a lower number of infected animals post challenge and a rapid elimination of the protozoa of the reproductive tract. These results allow us to present an alternative experimental model and inexpensive to evaluate power test of current commercial vaccines and new developments of immunogens against bovine trichomonosis
Key words: Tritrichomonas foetus; Mouse model; Vaccine.
Modelo experimental para evaluar el efecto inducido por vacunas contra Tritrichomonas foetus
Resumen
El objetivo del trabajo fué presentar un modelo experimental alternativo al bovino, para evaluar la eficacia de vacunas experimentales contra Tritrichomonas foetus. Las vacunas ensayadas se formularon con protozoários inactivados y diferentes adyuvantes: (A) con adyuvante oleoso, (B) vacuna acuosa, (C) vacuna acuosa con dimetilsulfósido. Se utilizaron 70 ratones hembras BALB/c distribuídos en 7 lotes experimentales; cada lote recibió 2 dosis de vacuna, dos lotes recibieron un booster vaginal al momento de la segunda dosis. Quince días posteriores a la segunda dosis, todos los animales fueron desafiados via vaginal con T. foetus. Muestras de sangre y mucus vaginal fueron tomadas hasta el día 30 pos infección. La respuesta inmune fue evaluada mediante un test de ELISA y la infección vaginal por cultivo de mucus vaginal en médio TYM. La mayor respuesta inmune se relacionó con un menor número de animales infectados pos desafío y una rápida eliminación de los protozoarios del tracto reproductor. Estos resultados nos permiten presentar un modelo alternativo y económico para evaluar las pruebas de potencia de las actuales vacunas comerciales y los nuevos desarrollos de inmunógenos contra la trichomonosis bovina.
Palabras clave: Tritrichomonas foetus; Modelo ratón; Vacuna.
Introduction
Bovine trichomonosis is a venereal disease caused by a protozoan, Tritrichomonas foetus (T. foetus). The infection in the female occurs during the estrus period, when the protozoan colonizes the reproductive tract mucosa. It manifests clinically through reproductive failure resulting from histopathological changes, with different degrees of vaginitis, cervicitis, endometritis and placentitis19. This pathogenic process induces a response at both the immune system and reproductive tract levels, in some cases favouring the elimination of the protozoan before 4 or 5 months post infection 21, 23. However, some females remain infected for over a year after conceptus loss. These carrier cows are an infection source for the herd 15, 20. The immune response to natural infection in cattle, as well as the refractoriness to reinfection observed in some animals, indicates that preventive immunization could be an alternative tool within a control program. The first scientific papers on vaccines against trichomonosis in cattle were published by Kerr, Robertson and Morgan 13, 14, 17. Several authors currently continue that line of research, with assays using different immunogens such as whole protozoa and/or subcellular fractions 4, 5, 6, 7, 9, 10, 18, 22. Their encouraging results motivated the development of commercial vaccines intended for pre-breeding use in cows in the United States and Argentina, as a strategy to increase resistance or rapid clearance of infection, thus improving production rates.
The growing interest in the development of new immunogens and vaccine strategies requires an easy to use and a low cost experimental model for power and efficacy testing. Experiments carried out in laboratory animals have shown that the BALB/c mouse is the most suitable model for this purpose 25, 26. During the course of our research on the use of the BALB/c mouse as an experimental model, we have defined the minimum concentration of estrogen to synchronize estrus as well as the lowest doses of T. foetus for vaginal infection and the experimental reproduction of trichomonosis by monitoring pathogen dynamics with and without gestation 3, 16, 24. We have found changes in the local immune response at the maternal-fetal interface 28, as well as changes in the expression of uterine epithelial carbohydrates 29, and induction of apoptosis involved in the pathogenesis of the early embrionic death during infection with T. foetus 30. This research has enabled us to develop an experimental model comparable to the bovine one, suitable for further research on mechanisms of pathogenicity, immune response, and evaluation of the protection conferred by different immunogens.
Given the current importance of vaccine development for a control program of bovine venereal trichomonosis, the aim of this study was to present the mouse model as an alternative to the bovine model, to evaluate the immunogenic efficacy of different formulations and routes of administration of inactivated T. foetus vaccines.
Materials and Methods
Vaccine
The vaccine was experimentally developed with a strain of T. foetus isolated from bovine vaginal mucus. The strain was subsequently identified by PCR using the TFR1 and TFR2 primers common to the Trichomonadidae family, and the TFR3 and TFR4 primers, specific for T. foetus 11.
The strain was cultured in TYM broth 8 and centrifuged at 1500 g during 20 minutes, and then the sediment was washed twice with sterile PBS pH 7.2. Subsequently, the sediment was resuspended in PBS with 0.1% formalin (v/v) at a concentration of 5x107 T. foetus/ml. Three types of vaccines were prepared with this suspension of inactivated protozoa:
- A: oil vaccine, consisting of one volume of T. foetus suspension, plus an equal volume of oil adjuvant (Sigma-Aldrich # F5506). - B: aqueous vaccine without adjuvant, consisting of one volume of T. foetus suspension plus an equal volume of sterile PBS.
- C: aqueous vaccine with DMSO, consisting of one volume of the T. foetus suspension plus one volume of PBS with 2% dimethyl sulfoxide (v/v) (Sigma-Aldrich).
Animals and vaccination schedule
Seventy 6-8 week old female BALB/c mice were used, divided into seven experimental groups of 10 animals each. Mice were bred at the animal facility of the Faculty of Veterinary Sciences, UNCPBA, and were housed according to national regulations 2 with controlled temperature and air flow, and ad libitum food and water. Lighting was provided on a 12-h light/dark cycle.
The groups received the following vaccination schedule. Group 1; two subcutaneous doses of vaccine A. Group 2; two subcutaneous doses of vaccine B. Group 3; two doses of vaccine B by vaginal instillation. Group 4; two doses of vaccine C by vaginal instillation. Group 5; two subcutaneous doses of vaccine A, plus one dose of vaccine B by vaginal instillation at the moment of the second subcutaneous dose. Group 6; two subcutaneous doses of vaccine B, plus one dose of vaccine B by vaginal instillation at the moment of second subcutaneous dose.
Group 7; unvaccinated control.
There was a 15 day interval between doses. Dose volumes of 200 and 20 μl were used for the subcutaneous and vaginal instillation routes, respectively
Experimental protocol schedule
The experiment was carried out according to the following schedule. Day 0; first vaccine dose. Day 15; second vaccine dose, plus one dose of vaccine B by vaginal instillation at the moment of the second subcutaneous dose for group 5 and 6. Day 28; estrus synchronization. Days 30 and 31; verification of estrus by vaginal cytology and infection by vaginal instillation of 10 μl of PBS with 9x105 T. foetus.
Blood and vaginal mucus samples were taken during the experimental period and until day 60 (day 30 post-infection), to assess the immune response both at a systemic level and in the reproductive tract, using ELISA and monitoring the vaginal infection by culturing of vaginal mucus in TYM broth.
Estrus synchronization
Mice from all groups were inoculated intramuscularly with 5 μg of b-estradiol 3-benzoate (SYNTEX S.A.) suspended in 0.1 ml of sterile sesame oil 24. In order to test the evolution of the estrus cycle, each mouse was examined by means of vaginal cytologic smears 24 and 48 hours later. The stage of the estrous cycle was determined based on the presence or absence of leukocytes, cornified epithelial cells, and nucleated epithelial cells. When the female is in proestrus, mostly nucleated and some cornified epithelial cells are present, and as the cycle advances to estrus, mostly cornified epithelial cells are observed 1.
Vaginal inoculum
The T. foetus strain was cultured in TYM broth, centrifuged at 1,500 g for 20 minutes and the sediment washed twice with sterile PBS at pH 7.2. Finally, the protozoa were resuspended into PBS and their concentration adjusted for the vaginal inoculum. When the estrus stage was determinated, all mice were inoculated intravaginally with 9x105 T. foetus in 10 μl. A second inoculation of T. foetus was given similarly the next day 24.
Sample collection
Blood collection was performed at the beginning of the experimental test and every 15 days until completion, by puncturing the submandibular venous sinus.
Vaginal mucus was obtained weekly from the first week post infection. The extraction was performed using a micropipette with disposable tips, instilling in the vagina 5 μl of PBS prior to aspiration, and the collected mucus was suspended in TYM broth. Additionally, vaginal mucus was obtained every 15 days by instillation and collection of 20μl of PBS. This was divided into an aliquot for protozoan culture in TYM and another aliquot for the ELISA determination of antibody titer.
Vaginal infection
To determine vaginal infection, each vaginal sample obtained was subjected to microscopic observation. The presence or absence of T. foetus was recorded. Samples were then cultured in TYM medium, to confirm the vaginal infection by means of protozoa isolation. When three consecutive weekly samplings showed negative results from both direct observation and culture, the mice were considered free of vaginal infection 24.
Evaluation of the immune response
In order to evaluate the induced immune response in this experimental model, and considering the small volume of the samples obtained the level of IgG in serum and IgA in vaginal mucus used as reference variables for this study.
Quantification of T. foetus antibodies was performed using an indirect ELISA, as previously described 7 with modifications for the mouse model. In brief, 96 well plates (Nunc-Immuno Plate PolySorp® surface) were pre-coated with 100μl in each well of whole T. foetus in 0.1 M carbonate buffer (pH 9.6) at a concentration of 1x106/ml. After 4 hours of incubation at room temperature, the wells were emptied and sealed plates were stored at - 20°C until analysis. Immediately before analysis, plates were blocked with 200 μl of PBS containing 3% gelatin and 0.02% sodium azide per well for 1 h at 37°C. Subsequently, they were washed 3 times with PBS containing 0.05% Tween 20 (PBS-Tw). Sera and vaginal mucus samples were diluted 1:100 and 1:10, respectively, with PBS containing 0.05% Tween 20 and 0.2% gelatin (PBS-Tw-G), and 100 μl of each sample was placed in the well and incubated at 37°C for 2 h. After washing the plates with PBS-Tw, 100 μl of mouse total anti IgG antibody obtained from goat conjugated with peroxidase (SIGMA # A4416) was added to each plate well containing a serum dilution, while 100 μl of mouse anti IgA obtained from goat conjugated with peroxidase (Sigma # A4789) was added to each well containing a diluted sample of vaginal mucus. Each antibody was previously diluted 1:2000 with PBS-Tw-G. After 30 minutes incubation at 37° C, the plates were washed again 3 times with PBS-Tw and 100 μl of substrate (ABTS-SIGMA) was added and incubated at room temperature in darkness for 40 minutes. The reaction was stopped by adding 100 μl per well of 1.5 M sodium azide. Optical density was read at 405 nm in an ELISA reader (Multiskan Ex - Labsystems).
All the samples were done in duplicate, and positive and negative control samples were included in each plate. As positive control, serum from ten mice subcutaneously immunized with formalized T. foetus were used. As negative control, serum samples were collected from non-infected mice. In both replications performed with the control sera and the experimental samples, the results obtained were stable.
Statistical analysis
The optical density (OD) in serum and in vaginal mucus was analyzed as the principal variable, using the PROC MIXED procedure of SAS V9.3 under a model that took into account the effect of batches and repeated measurements. The presence or absence of "vaginal infection" was similarly analyzed, using the GENMOD PROC procedure (SAS V9.3).
Results
Vaccination did not cause adverse general reactions or clinical signs in any of the different experimental groups. Upon the vaginal instillation challenge with T. foetus, most of the animals were in estrus and proestrus, due to the effect of the treatment with estradiol benzoate. After the first week post infection, the estrogenic effect decreased, allowing regular cycles throughout the test.
Vaginal infection. From microscopic direct observation, the mice whose vaginal mucus showed mobile protozoa with typical T. foetus morphological characteristics were considered infected. Negative samples from microscopic observation were cultured in TYM medium at 37°C for 7 days and observed microscopically every 24 hours. This methodology allowed us to determine the number of infected animals in each experimental group.
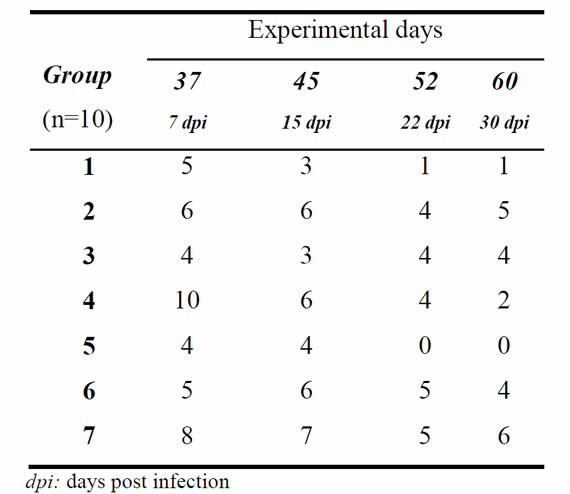
Animal infection. On day 7 post infection (day 37 of the experiment), the number of infected animals ranged from 40 to 100% among different groups, and by the end of the experiment a different outcome was observed between groups (Table 1). Analysis of the presence or absence of infection as a variable showed a significant effect of group (p=0.0016). Two clusters emerged from the comparison between groups at the end of the trial (day 60) The first cluster consisted of groups 1, 4 and 5, all with low infection rates that did not differ between them (p>0.05), with a percentage of infected animals at day 30 post infection (day 60) of 10%, 20% and 0%, respectively. The other cluster, consisting of groups 2, 3, 6 and 7, had higher infection rates, but which also did not show any differences between them (p>0.05), with infection rates at day 30 post infection (day 60) of 50%, 40%, 40% and 60%, respectively. The infection rate in the two clusters was statistically different (p<0.05).
Table I: Number of infected animals in each group at given experimental days after vaginal instillation with T. foetus.

Serum Immunoglobulin G (IgG) levels. Table 2 (section a) shows averages and standard errors of the OD for serum IgG levels for each experimental group at different sampling days. Measurement of the serum immune response allowed us to observe that groups 1, 2, 5 and 6 showed a rapid systemic response to vaccination, with a level of antibodies (Ab) that lasted until the end of the trial. No systemic response was detected in groups 3 and 4; these groups presented the same pattern as the control group (Figure 1). This different behavior over time for the different groups was observed from 15 days after the first vaccination onwards (p˂0.0001). On days 30, 45 and 60, two significantly different clusters were formed (p<0.05); a cluster with groups 1, 2, 5 and 6, with similar IgG levels between them (p>0.05), and a second cluster of groups 3, 4 and 7, with IgG levels similar to each other (p>0.05) but different to the first cluster.
Table 2: Means and standard errors of Optical Density of IgG in serum and IgA in vaginal mucus, for each group at different sampling days (n=10)
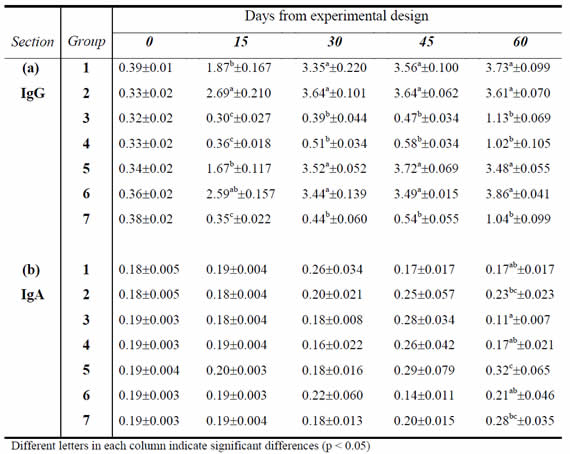
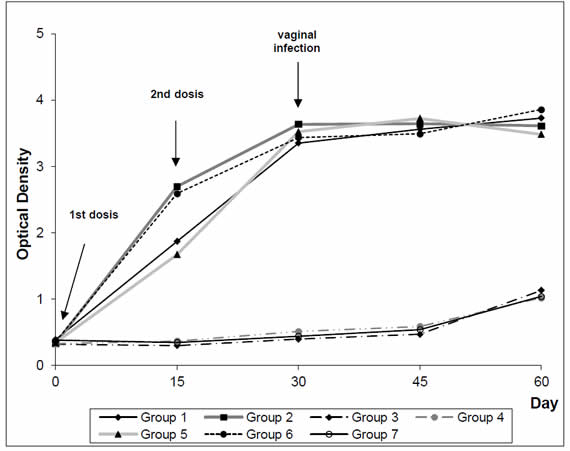
Figure 1. Serum IgG dynamics in female BALB/c vaccinated with T. foetus
Levels of Immunoglobulin A (IgA) in vaginal mucus. The average values and standard errors of the OD for levels of IgA in vaginal mucus are shown in Table 2 (section b) for each experimental group at different sampling days. Antibody levels in vaginal mucus were lower than the systemic response, as demonstrated by OD values very close to baseline levels. However, it was noted that groups 1, 2 and 6 had slightly higher values than the rest of the experimental and control groups at the time of vaginal challenge (day 30 post-first dose of vaccine). At 30 days post infection (day 60), group 3 had the lowest average level of OD, which was significantly different from groups 2, 7 and 5 (p<0.05).
Discussion
In the present study we use the BALB/c mice as an alternative experimental model to evaluate the immune response and efficacy of different formulations and administration routes of T. foetus vaccine. According to animal welfare criteria, the approval of the experimental design by the Faculty of Veterinary Sciences was based on use of the minimum sample volumes required for accurate analysis, and least invasive methodology. Accordingly, the sample volumes were defined during the investigation as the minimum necessary.
The ELISA test allowed us to evaluate the dynamics of the immune response based on the measurement of IgG in serum and IgA in vaginal mucus. Antibody level was considered as a study variable for this experimental design, and therefore it was not necessary to establish a cut off for this test.
Subcutaneous administration of vaccines A and B, with and without adjuvant, (groups 1 and 2, respectively), generated a similar level of serum antibody. This treatment plus the addition of the vaginal booster for groups 5 and 6, using the aqueous vaccine B, generated the same level of serum antibody than groups 1 and 2. However, in the groups immunized by vaginal instillation of vaccine B and C (group 3 and 4, respectively), serum antibody induced by the immunogen were not detected.
When the antibody level in vaginal mucus from different groups was analyzed, it was observed that OD values at the time of challenge by vaginal infection were very close to baseline levels obtained from the control group. At the end of the trial, little variation was observed in the antibody levels, with OD values ranging between 0.17 and 0.32 for the different groups.
The vaccine formulation C with DMSO was used in order to increase the permeability of the vaginal epithelium and evaluate the effect of this in stimulating the immune response. The application of this vaccine in group 4 did not generate serum antibody and the OD values were similar to those of group 3 (vaccine B) and group 7 (control). In these groups, an increase in the mean OD values was observed at the end of the trial as a response to vaginal infection by challenge with T. foetus.
In assessing the effect of the vaccine on the establishment of infection in animals of different groups, it was observed that the greatest protective effect was achieved in groups 1 and 5 (Table 1). Group 1 (vaccine A) showed 5 infected animals (50%) at day 7 post infection (day 37), and 1 infected animal (10%) at day 30 post infection (day 60). Group 5 (vaccine A, plus vaginal booster with vaccine B) had 4 infected animals (40%) at day 7 post infection (day 37), and all animals were negative from day 22 post infection (day 52) onwards (Table 1). In the control group, 80% of the animals were infected at day 7 post infection and 60% remained infected at day 30 post infection (day 60) (Table 1). Although groups 2 (vaccine B) and 6 (vaccine B plus vaginal booster with vaccine B) showed a good level of serum antibody, they completed the trial (day 60) with 50 and 40% of infected animals, respectively (Table 1). Variations in the number of infected animals in the different groups are responses to the effect induced by the immunogens tested, the conditions of each animal and the physiological state after estrogen treatment. In this context, the significant decrease in the number of infected animals in the vaccinated groups corresponds to the immune response induced by the vaccines applied, the type of adjuvant and route of application.
From the analysis of the results it is therefore possible to conclude that the subcutaneous administration route generates a systemic immune response with a high level of antibodies. This response helps to reduce the number of infected animals and a rapid removal of protozoa from reproductive tract. Vaccination by vaginal instillation with the two tested immunogens does not generate an immune response. The use of subcutaneously-administered vaccine with oil adjuvant plus a vaginal booster produces the greatest protective effect against the vaginal challenge with T. foetus. Although the vaginal application of the immunogen as a booster improves the IgA response in vaginal mucus, it is not a suitable on-farm practice.
The use of vaccines as part of a bovine trichomonosis control program has seen an increased interest in countries where natural breeding is part of the cattle production cycle. Given that T. foetus is an extracellular parasite, the antibody response is very important in the defense mechanism to eliminate the protozoa from the reproductive tract. Serum, vaginal and uterine antibodies have been already studied during the infection in cattle 4, 21, 23. Commercially-available whole protozoa vaccines elicit an immune response that shortens the genital infection period and reduces reproduction failures 7, 10, 18. T. foetus subunit vaccines have also been tested; these vaccines contain solely a protozoan subunit selected for its antigenic capability to induce an efficient immune response. Certain superficial antigen subunits such as Tf190 and Tf1.17 seem to be good vaccine candidates 4, 12, 27. The inroads of molecular biology together with a deep knowledge of the pathogenesis of T. foetus are opening new fields of research for the design of experimental vaccines, which could in turn improve the performance of current ones.
Again this background, the results of our research encourage the use of a low-cost, alternative experimental model in future studies on immune response and efficacy of new vaccines prior to their validation in cattle.
1. Allen, E. The estrous cycle in the mouse. Am J Anat; 1922; 30:297.
2. ANMAT. Reglamentación para Bioterios de Laboratorios elaboradores de Especialidades Medicinales y/o Análisis de terceros. Cap. I, II, III, IV, V. Disposición 6344. Administración Nacional de Medicamentos, Alimentos y Tecnología Médica. Argentina. 1996.
3. Barbeito, C.; Woudwyk, M.; Cacciato, C.S.; Soto, P.; Portiansky, E.; Catena, M.; Echevarría, H.M.; Gimeno, E.; Monteavaro, C.E. Tritrichomonas foetus: Experimental infection in pregnant BALB/c mice. Exp Parasitol; 2008; 120:156-160.
4. BonDurant, R.H. ; Corbeil, R.R. ; Corbeil, L.B. Immunization of virgin cows with surface antigen. Tf1.17 of Tritrichomonas foetus. Inf and Imm; 1993; 61(4):1385-1394.
5. Campero, C.M.; Medina, D.; Rossetti, O.; Marcovecchio, F.; Cosentino, B.; Marcone, J.; Carracino, M. Vacunación subcutánea e intravaginal contra tricomonosis en vaquillonas. Rev Med Vet. 1998; 79(5):347-352.
6. Clark, B.; Emery, D.L.; Dufty, J.H. Therapeutic immunization of bulls with the membranes and glycoproteins of Tritrichomonas foetus var. Brisbane. Aust Vet J. 1984; 61(2)65-66.
7. Cobo, E.R.; Morsella, C.; Cano, D.; Cipolla, A.; Campero, C.M. Immunization in heifers with dual vaccines containing Tritrichomonas foetus and Campylobacter fetus antigens using systemic and mucosal routes. Theriogenology. 2004; 62:1367-1382.
8. Diamond, L.S. Lumen-dwelling protozoa: Entamoeba, Trichomonads and Giarda. In vitro cultivation of Protozoan Parasites. In: Jensen J.B. (ed), CRC Press, Boca Ratón, Florida. 1983; pp. 89-98.
9. Edmonson, M.A.; Joiner, K.S.; Spencer, J.A; Ridell, K.P.; Rodning, S.P.; Gard, J.A.; Givens, M.D.. Impact of a killed Tritrichomonas foetus vaccine on clearance of the organism and subsequent fertility of heifers following experimental inoculation. Theriogenology. 2017; 90:245-251
10. Fuchs, L.I.; Fort, M.C.; Cano, D.; Bonetti, C.M.; Gimenez, H.D.; Vazquez, P.M.; Bacigalupe, D.; Breccia, J.D.; Campero, C.M.; Oyhenart, J.A.. Claerance of Tritrichomonas foetus in experimentally infected heifers protected with vaccines based on killed-T foetus with different adjuvants. Vaccine. 2017; 35:1341-1346.
11. Felleisen, R.; Lambelet, N.; Bachmann, P.; Nicolet, J.; Müller, N.; Gottstein, B.. Detection of Tritrichomonas foetus by PCR and DNA enzyme immunoassay based on rRNA gene unit sequences. J Clin Microbiol. 1998; 36:513-519.
12. Hodgson, J.L.; Jones, D.W.; Widders, P.R.; Corbeil, L.B. Characterization of Tritrichomonas foetus antigens by use of monoclonal antibodies. Infec. Imm. 1990; 58:3078-3083.
13. Kerr, W. Trichomoniasis in the cow. Vet Jour. 1943; 99:145-151.
14. Kerr, W.; Robertson, M. Experimental infections in virgin heifers with Trichomonas foetus in vaccinated and unvaccinated animals. J of Comp Path. 1946; 56:101-103.
15. Mancebo, O.A.; Russo, A.M.; Carabajal, L.L.; Monzon, C.M. Persistente of Tritrichomonas foetus in naturally infected cows and heifers in Argentina. Vet Parasitol. 1995; 59:7-11.
16. Monteavaro, C.E.; Aguirre, J.I.; Soto, P.; Echevarría, H.M.; Catena, M.C.; Portiansky, E.L.; Gimeno, E.J. Interaction of Tritrichomonas foetus with the reproductive tract of experimentally infected female BALB/c mice: ultrastructural evaluation. The Vet J. 2007; 173:206-210.
17. Morgan, B. Vaccination studies on bovina trichomoniasis. Am J Vet Res. 1947; 8:54-56.
18. Palomares, R.A.; Hurley, D.J.; Crum, L.T.; Rollin, E.; Collop, T.; Williard, A.; Felton, J.; Parrish, J.; Corbeil, L.B. Serum, uterine, and vaginal mucosal IgG antibody responses against Tritrichomonas foetus after administration of a commercial killed whole T foetus vaccine in beef cows. Theriogenology. 2017; 87:235-241.
19. Parsonson, I.M.; Clark, B.L.; Dufty, J.H. Early Pathogenesis and Pathology of Tritrichomonas foetus infection in virgin heifers. J Comp Pathol . 1976; 86:59-66.
20. Skirrow, S. Identification of Trichomonad-carrier cows. J Am Vet Med Assoc. 1987; 191:553-554.
21. Skirrow, S.Z.; BonDurant, R.H. Immunoglobulin isotype of specific antibodies in reproductive tract secretion and sera in Tritrichomonas foetus infected heifers. Amm J Vet Res. 1990; 51:645-657.
22. Soto, P.; Lucchesi, E. Tratamiento inmunológico de la trichomoniasis genital en toros infectados con cepas quimioresistentes. Rev Vet Arg 1986; 30:980-986.
23. Soto, P.; Parma, A.E. The immune response in cattle infected with Tritrichomonas foetus. Vet Parasitol. 1989; 33:343-348.
24. Soto, P.; Echevarría, H.; Monteavaro, C.; Catena, M. Experimentally induced intravaginal Tritrichomonas foetus infection in mouse model. Pes Vet Bras. 2005; 25(4)225-230.
25. St Claire, M.C.; Riley, L.K.; Franklin, C.L.; Besh-Williford, C.L.; Hook, R.R. Jr. Experimentally induced intravaginal Tritrichomonas foetus infection in the estrogenized mouse. Lab Anim Sc. 1994; 44(5):430-435.
26. VanAndel, R.A.; Frankiln, C.L.; St Claire, M.C.; Riley, L.K.; Besh-Williford, C.L.; Hook, R.R. Jr. Lesions of experimental genital Tritrichomonas infections in estrogenized BALB/c mice. Vet. Pathol. 1996; 33:407-411.
27. Voyich, J.M.; Ansotegui, R.; Swenson, C.; Bailey, J.; Burges, D.E. Antibody responses of cattle immunized with Tf 190 adhesin of Tritrichomonas foetus. Clinical and Diagnostic Laboratory Immunology. 2001; 8(6):1120-1125.
28. Woudwyk, M.A.; Monteavaro, C.E.; Jensen, F.; Soto, P.; Barbeito, C.G.; Zenclussen, A.C. Study of the uterine local immune response in a murine model of embryonic death due to Tritrichomonas foetus. Am J of Repr Imm. 2012; 68(2):128-137.
29. Woudwyk, M.A.; Gimeno, E.J.; Soto, P.; Barbeito, C.G.; Monteavaro, C.E. Lectin binding pattern in the uterus of pregnant mice infected with Tritrichomonas foetus. J Comp Path. 2013; 149:341-345.
30. Woudwyk, M.A.; Zanuzzi, C.N.; Nishida, F.; Gimeno, E.J.; Soto, P.; Monteavaro, C.E.; Barbeito, C.G. Apoptosis and cell proliferation in the mouse model of embryonic death induced by Tritrichomonas foetus infection. Exp Parasitology. 2015; 156:32-36.