ARTÍCULO DE INVESTIGACIÓN
Different strategies for the diagnosis of ovine brucellosis by brucella ovis in an endemic flock from argentina
Fiorentino, M.A.1; Velilla, A.1; Manes, J.2; Díaz, A.G.3; Clausse, M. 3; Paolicchi, F.A.1 & Estein, S.M.3
1Laboratorio de Bacteriología, Departamento de Producción Animal, Estación Experimental Agropecuaria (EEA) Balcarce Instituto Nacional de Tecnología Agropecuaria (INTA).
2Biotecnología de la Reproducción, Departamento de Producción Animal, EEA Balcarce, INTA, Balcarce, Argentina.
3Laboratorio de Inmunología, Departamento SAMP, Centro de Investigación Veterinaria de Tandil (CIVETAN)-CONICET-CIC, Facultad de Ciencias Veterinarias, Universidad Nacional del Centro de la Provincia de Buenos Aires (UNCPBA)Facultad de Ciencias Veterinarias, Universidad Nacional de La Pampa
Recibido: 08/10/2017
Aceptado: 10/04/2018
Correspondencia e-mail: Silvia Estein silviamestein@gmail.com
Diferentes estrategias para el diagnóstico de la brucelosis ovina por brucella ovis en una majada endémica de Argentina
Summary
Brucella ovis is the etiological agent of contagious epididymitis in rams. Prevalence increases when biannual control was not performed in B. ovis endemic flocks. Diagnosis is based on clinical examination, serological tests and bacteriological isolation of B. ovis from semen. Semen analysis by PCR technique complements the direct diagnosis for the detection of B. ovis or other reproductive pathogens in sheep. In this work, different strategies for the diagnosis of contagious epididymitis in a flock, which this disease has been apparently controlled, were compared. Twenty-two rams were clinically examined and serum samples were analyzed by different serological tests. Semen samples were cultured in bacteriological media and were evaluated by multiplex PCR. Rams with a positive culture were necropsied and testes, epididymis, vesicular glands and ampullae were taken and studied by histopathology. B. ovis shedding in semen was significantly associated with the seropositivity and genital tract histopathological lesions. Multiplex PCR showed a similar sensitivity to semen culture and could be used as a complementary test for the direct diagnosis of B. ovis. Although this study involved a low number of animals, it provides useful information for the diagnosis of ovine brucellosis in an endemic flock and reinforces the need for biannual control.
Key words: Brucella ovis; Ram epididymitis; Diagnosis; Control.
Resumen
Brucella ovis es el agente etiológico de la epididimitis contagiosa del carnero. El diagnóstico se basa en el examen clínico, la serología y en el aislamiento de B. ovis a partir de semen. El análisis del semen mediante PCR complementa el diagnóstico directo para la detección de B. ovis y de otros patógenos reproductivos de los ovinos. En este trabajo se comparan diferentes estrategias para el diagnóstico de la epididimitis contagiosa del carnero. Veintidós carneros fueron examinados clínicamente. Las muestras de suero fueron analizadas con diferentes pruebas serológicas. Las muestras de semen se cultivaron y evaluaron mediante PCR multiplex. Los carneros con cultivo positivo fueron sacrificados y se tomaron muestras de testículos, epididídimos, vesículas seminales y ampollas del conducto deferente para el estudio por histopatológico. La presencia de B. ovis en semen se asoció significativamente con la seropositividad y con lesiones histopatológicas en los órganos estudiados. La multiplex PCR mostró una sensibilidad similar al cultivo de semen y podría ser usada para el diagnóstico directo de B. ovis. Aunque este estudio fue realizado en pocos animales, este provee información útil para el diagnóstico de la brucelosis ovina en una majada endémica y refuerza la necesidad de dos controles por año.
Palabras clave: Brucella ovis; Epididimitis del carnero; Diagnóstico; Control.
Introduction
Brucella ovis is the etiological agent of contagious ram epididymitis1. This chronic disease causes significant economic loss in sheep breeding farms associated with the slaughter of reproductive males with genital lesions (epididymitis and orchi-epididymitis) 2. Besides the reduced fertility in rams, it occasionally causes embryonic mortality, abortion or infertility in ewes and increased perinatal mortality in lambs.(1,3). Passive venereal transmission via the infected ewe appears to be the most frequent route of infection, but ram-to-ram transmission is also very common 3. Direct ram-to-ram transmission during non-breeding periods is thus quite frequent and has been suggested to take place by several routes, such as through oral-genital contact (licking or sniffing infected semen) or by rectal copulation (4).
The approach to the control of B. ovis depends on flock and farm characteristics, disease prevalence, and economic factors. In flocks where eradication and prevention of reintroduction are feasible, control is based on serological test and slaughter 5. Nowadays, the live attenuated vaccine B. melitensis Rev. 1 is the only effective way to control B. ovis infection in areas with a high or moderate prevalence where eradication would be difficult. However, this vaccine displays a large number of shortcomings, including residual virulence, pathogenicity for humans and interferences with serodiagnosis which limits widespread use worldwide 3. In Argentina, National Food Safety and Quality Service (SENASA) only authorizes the vaccination of sheep that coexist with goats in areas where the caprine brucellosis is endemic.
The demonstration of genital lesions (unilateral or bilateral epididymitis and orchi-epididymitis) by scrotal palpation of rams may suggest the presence of this infection in a given flock. However, clinical diagnosis lacks sensitivity because not all rams infected with B. ovis show palpable genital lesions 1. Moreover, clinical diagnosis lacks specificity since many other bacteria may cause genital lesions in rams. The most frequently reported pathogens causing such lesions in rams include Actinobacillus seminis and Histophilus ovis 6. For this reason, diagnosis confirmation is based on direct or indirect laboratory examination. Direct diagnosis is made by means of bacteriological isolation of B. ovis from semen samples or vaginal discharges, udder secretions and tissues of ewes, on adequate selective media. However, shedding of B. ovis in fluids can be intermittent and these organisms are fastidious, therefore, a single negative sample does not guarantee that an animal is negative. Molecular methods have been developed for complementary identification based on specific genomic sequences. Therefore, Polymerase chain reaction (PCR) based methods can provide an additional tool to complement direct diagnosis of B. ovis and multiplex-PCR technique can be successfully used for the detection of three of the most common bacterial causes of ovine epididymitis.(7, 8, 9).
Indirect diagnosis based on serological tests is preferred for routine diagnosis. Currently, the most widely used tests are Agar Gel Immunodiffusion (AGID), complement fixation (CFT) and indirect ELISA (iELISA), in which the antigen is a hot-saline extract of B. ovis (HS) (1,10). In addition, B. canis (M-) strain that shares epitopes with B. ovis used in Rapid Slide Agglutination Test without and with2 Mercaptoethanol (RSAT o 2ME-RSAT, respectively) and HS obtained from this strain is used in ELISA (7,11).
The aim of this study was to evaluate direct and indirect diagnosis strategies for ovine brucellosis in an endemic flock from Buenos Aires Province (Argentina) where a single cull based on clinical examination and serological test was ineffective in decreasing the prevalence of B. ovis.
Materials and methods
Animals
This study was carried out on a sheep farm located in Ayacucho (Buenos Aires province, Argentina). The farm had 670 Corriedale and Romney Marsh sheep: 560 ewes and 110 males (88 lambs and 22 rams). System production was extensive (self-replacing) and based on the use of natural pastures throughout the year. Only rams were supplemented with maize-corn concentrate.
Historically, the owner of this flock had been testing biannually by clinical examination and serological tests for ovine brucellosis for 5 years and no evidence of B. ovis infection had been found. However, two years earlier ram epididymitis by B. ovis had been recorded in this flock. Thirty percent of seropositive rams had been discarded. However, the previous year, rams had not been examined and had not been analyzed by serology. To start the study, the farmer pointed out that the disease had been controlled, without reproductive losses (91% of pregnancy, 110% of calving and 110% of weaning).
Clinical examination
Twenty-two Corriedale/Romney Marsh rams were evaluated by palpation of cranial (submandibular) and inguinal lymph nodes and scrotal contents to detect testes and epididymis lesions according to Jackson and Cockcroft (2002)12. Differences in size, consistency, shape or swellings in external genitalia were considered abnormal and were registered. In addition, prepuce and penis were inspected to detect abnormalities that could affect the reproductive performance of animals.
Samples
Animal procedures and management protocols were approved by the Ethics Committee according to the Animal Welfare Policy (act 087/02) of the Faculty of Veterinary Medicine, Universidad Nacional del Centro de la Provincia de Buenos Aires (U.N.C.P.B.A), Tandil, Argentina (http://www.vet.unicen.edu.ar).
Blood samples were collected from each ram by jugular venipuncture for serological analysis. Semen samples were obtained by electroejaculation (see Semen collection) from rams with genital lesions and positive or suspected serological reactions. These rams were necropsied and testes, epididymis, vesicular glands and were taken for bacteriological and histopathological studies
Semen collection
Semen samples for bacteriological culture and multiplex polymerase chain reaction (PCR) were obtained by electroejaculation with EE Electrojac V® stimulator (Ideal instruments, Neogen Company, Lansing MI, USA) with a rectal probe 22 cm long, 2.5 cm in diameter and three lineal electrodes. Animals received an intramuscular injection of xylazine (0.2 mg/kg body weight; 2%; Rompun, Bayer S.A., Argentina). The rectal probe was lubricated and gently inserted into the rectum and orientated so that the electrodes were positioned ventrally. The device was used in the automatic setting, applying cycles of stimuli of 2 s with 2-s rest intervals between stimuli. Voltage was increased by one cycle (0.5 V) at a time. According to the individual animal sensitivity to electrostimuli, the minimum voltage required to obtain an ejaculate was used, without exceeding the seven cycles. The penis was extended beyond the prepuce, and semen was collected into a sterile collection tube. Samples were refrigerated to be transported to the lab.
Serological tests
Buffered Plate Agglutination Test (BPAT)
BPA test was performed to evaluate serological interference induced against smooth Brucellae. Antigen was purchased from Laboratorio Biológico de Tandil (Tandil, Argentina). Results were interpreted according to the procedures recommended by SENASA 13.
Agar Gel Immunodiffusion Test (AGID)
AGID test was done following OIE instructions 10. The test was carried out in Petri dishes covered with a gel composed by 1 g of Noble agar dissolved in Borate buffer added with 10 g/% of NaCI. A gel puncher with six peripheral holes for control and test sera, and a central hole for antigen was used to cut the gel. The Heat-extracted B. ovis antigen (HS Bo) was provided by SENASA. Interpretation of results was made after incubation for 48 h at room temperature in a humid chamber.
Indirect Enzyme-Linked Immunoassay (iELISA) and B. canis agglutination test
Two tests were performed using antigens prepared with (M-) variant of this B. canis. Indirect ELISA against HS from B. canis was carried out as described previously 14.
B. canis agglutination test was performed mixing 10 μL of serum with 10 μL of antigen 14. The 2 Mercaptoethanol-RSAT (2ME-RSAT) was done by mixing 10 μl of serum with 10 μl of 2-ME solution (0.2 M). After 1 min, 20 μl of antigen was added and the reaction was read7. B. canis antigen was produced in Laboratorio de Inmunología, Facultad de Ciencias Veterinarias of the U.N.C.P.B.A. (Tandil, Argentina).
Culture of semen
Semen samples were seeded in blood agar Columbia (Oxoid Ltd, Wad Road, Basingstoke, UK) added with 7% of bovine defibrinated blood and in modified Skirrow media agar 15. Plates were incubated in 10% of CO2 at 37ºC for seven days. Suspected colonies were identified by Gram and Stamp staining, catalase, oxidase, urease and SH2 production 16.
Multiplex Polymerase Chain Reaction
Extraction of DNA
Bacterial cultures
To determine the specificity of the PCR, genomic DNA was extracted from pure cultures of B. ovis, Actinobacillus seminis e Histophilus somni strains isolated from clinical cases and also from the strain collection of Laboratorio de Bacteriología (EEA INTA Balcarce). Briefly, single colonies were suspended in 50 μL sterile ultrapure water and incubated in a 100°C heating block for 10 min. Lysates were centrifuged and the cell-free supernatant transferred to a fresh tube. DNA extracts were stored at -20°C.
Semen
DNA from semen samples were extracted using a commercial kit QIAmp® DNA mini Kit (QIAGEN, Hilden, Alemania) according to the manufacturer’s recommendations.
Multiplex PCR was performed using primers and reaction conditions previously described and adapted by Fiorentino et al. (2011) (17,18). Briefly, the PCR was performed in a final volume of 50 μL, containing 3 Unit of Go Taq DNA Polymerase (Promega, Madison, WI), 10 μL of 5x Buffer (Promega, Madison, WI), 2.5 mM de MgCl2, 200 μM each of forward and reverse primers, 0.2 mM dNTPs and 10 μL of template DNA.
Cycling conditions were denaturation at 94°C for 2 min, followed by 35 cycles of 94°C for 30 sec, 55°C for 30 sec and 72°C for 1 min, and a final extension of 72°C for 6 min. PCR was performed in a THERMO® Electron Corporation (Milford, USA) and PCR products were separated on a 1.0% agarose and stained with SyberSafe® (Invitrogen, USA).
Histopathology
Eight of the nine animals with clinical lesions were slaughtered and portions of epididymis and vesicular glands were fixed in 10% buffered formalin and embedded in paraffin. Sections 4-5 μm were cut and stained with hematoxylin and eosin by standard procedures.
Results
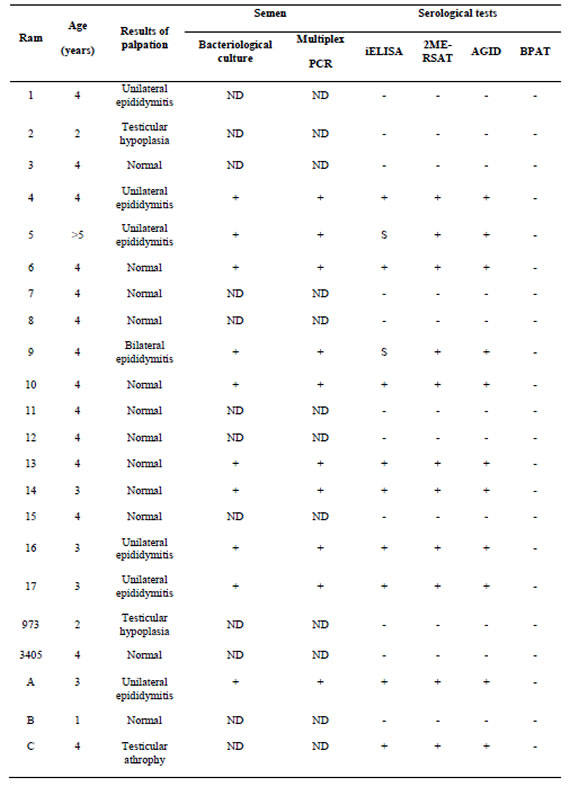
Comparison of the clinical, bacteriological, serological and PCR results of all rams are shown in Table 1. Ten rams (45.45%) presented scrotal palpable lesions. Seven of these rams showed the tail as the most frequently affected part of epididymis with unilateral rather than bilateral presentation Problems in the testes were minor and related to hypoplasia or atrophy (30%) with increased hardness. The rest of the animals (12/22) did not show any alteration during the clinical inspection. None of rams had enlarged or increased of inguinal lymph nodes.
Serum antibodies were studied by BPAT, AGID, 2ME-RSAT and iELISA. The comparative results of serological test are given in Table 1. All of the rams were found negative to BPAT, ruling out exposure to smooth LPS Brucella spp., while 10 animals were positive or suspicious for all serologic tests performed to study immune response against B. ovis (45.45%). For 2ME-RSAT and AGID tests, 10 rams were positive while 2/10 rams were suspicious in iELISA with titers around the cut-off limit. The results indicate a higher sensitivity of 2ME-RSAT/AGID test with respect to the iELISA used in our work. As shown in Table 1, serological positive and suspicious rams had lesions either in epididymis or testes. Semen culture and PCR were performed in 10 rams. Both techniques were positive in agreement with the presence of palpable lesions and positive or suspicious serological results.
The rams with palpable lesions and serological results were necropsied. Gross lesions were observed only in eight of the ten animals with clinical lesions. The lesions were severe in all animals and were characterized by interstitial edema and epithelial vacuolation, with areas of hyperplastic epithelium forming "intraepithelial cysts" (Figure 1), severe interstitial fibrosis and in some cases spermatic granulomas were also observed. Lesions in the vesicular glands and ampullae were characterized by mononuclear focal infiltrate ranging from mild to moderate as well as congestion and edema and epithelial hyperplasia (Figure 2).
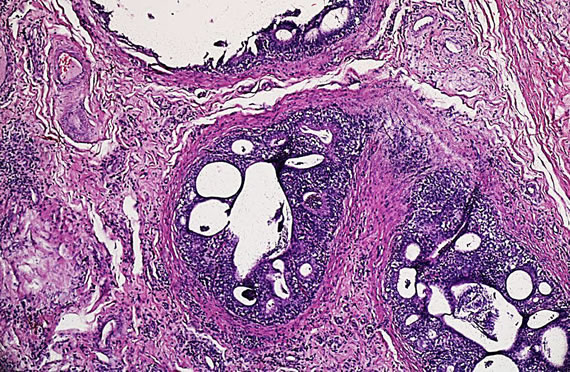
Figure 1. Characteristic microscopic lesions in the epididymis of Brucella ovis infected ram. Interstitial edema and inflammatory cells. Epidydimal ducts show epithelial hyperplasia and intraepithelial cysts with peritubular fibrosis and neovascularization (Hematoxilin-Eosin stain X1000).
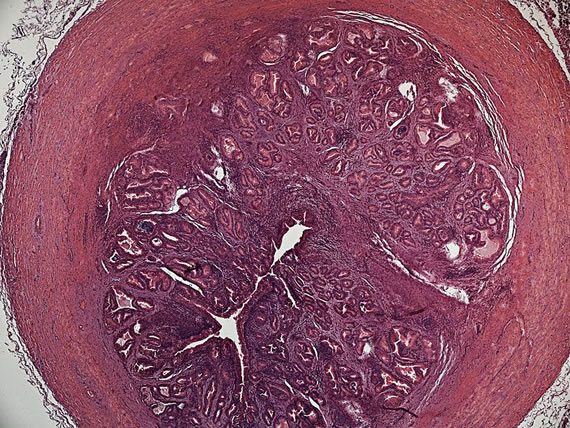
Figure 2. Ampulla of ductus deferens of Brucella ovis infected ram. Section through the ampulla showing severe subepithelial chronic inflammation with fibrosis and lymphoplasmatic infiltrate (Hematoxilin-Eosin stain X400).
Discussion
The only unequivocal method for the diagnosis of animal brucellosis depends on the isolation of Brucella spp.1. However, it has been generally accepted that the diagnosis of B. ovis infection can be made using clinical, bacteriological and serological criteria 5. In the present work, we evaluated different tools for direct and indirect diagnosis of ovine brucellosis in an endemic flock.
Scrotal palpation and serologic testing may indicate if B. ovis infection is present. The most efficient and widely used serological tests are AGID and iELISA with HS from B. ovis as antigen. Recently, RSAT and iELISA using antigens prepared with B. canis (M-) strain were proposed for diagnosing B. ovis infection in sheep. In our work, three tests gave positive or suspicious (ELISA) results in ten rams with palpable lesions and positive bacteriological and PCR diagnosis.
Due to the high occurrence of asymptomatic infections by B. ovis a clinical diagnosis is very difficult. According to Burgess (1982), shedding of B. ovis in the semen is considered the main source of infection in the flock 19. Therefore, semen is considered the sample of choice for detection of B. ovis 19. In our study, the isolation of B. ovis from semen samples was consistent with those previously reported in experimental infections by other authors 1. Ridler et al., (2014) observed that rams had been shedding B. ovis in semen for at least 4 weeks before antibodies were detected in their sera by AGID 21. Unfortunately, in our work, it was not possible to collect semen samples from serologically negative rams. However, all rams positive or suspicious of any of the serological tests performed had epididymal or testicular lesions. This situation could be due to the chronicity of the infection in the herd.
As previously stated, the isolation of B. ovis is the gold standard test. However, that organism is fastidious and slower growing than common contaminants of semen samples. Primary isolation and presumptive identification are often difficult and time-consuming. While a selective medium, such as modified Skirrow Agar can be used for the isolation of B. ovis, overgrowth by contaminants may still occur 15. In addition, there are other bacteria that can cause epididymitis in rams such as H. somni and A. seminis and no selective media are available for them (22, 23) .The limitations of culture have led to the development of PCR methodologies for the detection of these three pathogens. In our study, the multiplex PCR results showed concordance with a positive bacteriological culture of semen (10/10) and allowed the exclusion of other causes of epididymitis in rams. These results agree with those reported by Saunders et al. (2007) and other authors who used other primers for detection of nucleic acid fragments of B. ovis (23, 24). Since the extraction of semen can be laborious, some authors have proposed performing PCR from urine samples obtaining similar levels of sensitivity when compared to the culture or PCR of semen samples in naturally or experimentally infected animals (8).
The release of B. ovis from phagocytic cells and tissue contact causes a strong serological and cellular response, with the development of severe lesions that lead to the formation of spermatic granulomas. In our work, the histopathologic lesions in the epididymis and seminal vesicles were consistent with those described by other authors (20, 25) .
Nine of the ten animals with clinical signs presented gross lesions in epididymis at necropsy. However, incipient lesions cannot be detected by clinical inspection and antibodies appear more often and earlier (15-21 days) than clinical lesions (30-45 days) in animals experimentally infected 20. Recently, Carvalho Júnior et al., 2012 used the ultrasonography to detect the kinetic changes in the reproductive organs of rams experimentally infected with B. ovis but they concluded that they were not specific and demonstrated the need for laboratory confirmation under field conditions 26. In this regard, B. ovis infection was diagnosed by serology and bacteriology in four rams that appeared normal to clinical inspection 27. This is consistent with the observations made by Robles et al. (1998) who claim that the removal of animals with clinical lesions as a single measure of sanitation in endemic farms can reduce the clinical epididymitis but increases the serological prevalence of B. ovis 28. In this work, Robles et al. (1998) show clearly that when the criterion adopted to eradicate ovine brucellosis is just culled rams with clinical lesions, subclinical rams are excreting B. ovis, becoming potential spreaders of disease in the flock. Different authors have reported an increment in prevalence from 30 to 45% when biannual control was not performed in B. ovis endemic flocks (1,27). As it was observed in our study, a single cull based on the results of clinical examination and only one serological test was ineffective in decreasing the prevalence of B. ovis.
In summary, adequate knowledge of the epidemiology of ovine brucellosis, culling of the seropositive animals and those with genital lesions is of great importance to control this disease. Effective control measures are critical to avoid recurrence of ovine brucellosis.
On the other hand, in those flocks where ovine brucellosis is endemic, multiplex PCR could be incorporated as a complementary or alternative tool to the semen culture for the rapid detection of B. ovis and its differentiation from other possible causes of ovine epididymitis. Although this study involved a low number of animals, it can provide useful information for the diagnosis and control of ovine brucellosis in an endemic farm.
1. Blasco, J.M. 1994. Brucella ovis. In: Animal Brucellosis, Ed by Nielsen and Duncan. Boca Raton, Florida, USA.p 453-470.
2. Paolicchi, F.A.; Campero, C.M.; Zamora, A.S.; Cipolla, A.L.; Casaro, A.P. Lesiones anatomopatológicas en genitales de carneros enviados a faena. Vet. Med. Res. 1991; 72: 176-185.
3. Blasco, J.M.; Marín, C.M.; Barberán, M.; Moriyón, I.; Díaz, R. Immunization with Brucella melitensis Rev 1 against Brucella ovis infection of rams. Vet. Microbiol. 1987; 14:381-392.
4. Hartley, W.J.; Jebson, J.L.; McFarlane, D. Some observations on natural transmission of ovine brucellosis. N. Z. Vet. J. 1955; 3:5–10.
5. Ridler, A.L.; West, D.M. Control of Brucella ovis infection in sheep. Vet. Clin. North Am. Food An. Pract. 2011; 27:61–66.
6. Jebson, J.L.; Hartley, W.J.; McFarlane, D. The artificial infection of sheep with a Brucella-like organism. Part II: The artificial infection of rams. N. Z. Vet. J. 1954; 2:85–89.
7. Manterola, L.; Tejero-Garcés, A.; Ficapal, A; Shopayeva, G.; Blasco, J.M., Marin, C.M.; López-Goñi, I. Evaluation of a PCR test for the diagnosis of Brucella ovis infection in semen samples from rams. Vet. Microbiol. 2003; 92: 65-72.
8. Costa, EA; Sant’Ana, F.M.; Carvalho, C.J.S.; Moustacas, V.S.; Silva, S.M.M.S; Paixão, T.A.; Santos, R.L. Diagnosis of Brucella ovis infection by serology and PCR in urine samples from naturally infected rams in the State of Piauí. Arq. Bras. Med. Vet. Zootec. 2012; 64:751-754.
9. Xavier, M.N.; Silva, T.M.A.; Costa EA, Paixão TA, Moustacas VS, Carvalho CA Jr, Sant'Anna FM, Robles CA, Gouveia AM, Lage AP, Tsolis RM, Santos RL. Development and evaluation of a species-specific PCR assay for the detection of Brucella ovis infection in rams. Vet. Microbiol. 2010; 145:158-164.
10. OIE. 2015. Ovine epididymitis (Brucella ovis). Manual of Standards Diagnostic Tests and Vaccines. Office International des Epizooties. 5th ed. Paris. Chapter 2.7.9. p 1-9.
11. Carmichael, L.; Joubert, J. A rapid slide agglutination test for the serodiagnosis of Brucella canis infection that employs a variant (M) organism as antigen. Cornell Vet. 1987; 77:3-12.
12. Jackson, P.G.G.; Cockcroft, P.D. 2002. Clinical Examination of the sheep. In: Clinical Examination of Farm Animals, Blackwell Science Ltd., Oxford, UK. doi: 10.1002/9780470752425.chapter 15.
13. Nicola, A.; Elena S. 2009. Manual de Diagnóstico Serológico de la Brucelosis Bovina. SENASA, Buenos Aires. p. 95.
14. López, G.; Ayala, S.M.; Escobar, G.I.; Lucero, N.E. Use of Brucella canis antigen for detection of ovine serum antibodies against B. ovis. Vet. Microbiol. 2005; 105:181-187.
15. Terzolo, H.R.; Paolicchi, F.A.; Moreira, A.R.; Homse, A. Skirrow agar for simultaneous isolation of Brucella and Campylobacter species. Vet. Rec. 1991; 129:531-532
16. Alton, G.G.; Jones, L.M.; Angus R.D.; Verger, J.M. 1988. Serological methods. techniques for the brucellosis laboratory. Institut National de la Recherche Agronomique, Paris, France. p 157-167.
17. Fiorentino, M.A.; Velilla, A.; Manes, J.; Zimmer P, Salas J, Draghi MG, Paolicchi, F. 2011. PCR múltiple para el diagnóstico de epididimitis infecciosa ovina en Argentina. In Proceeding of Jornadas Internacionales de Veterinaria Práctica, Mar del Plata, Argentina.
18. Moustacas, V.S.; Silva, T.M.; Costa, L.F.; Xavier, M.N.; Carvalho, C.A. Jr, Costa, E.A.; Paixão, T.A.; Santos, R.L. Species-specific multiplex PCR for the diagnosis of Brucella ovis, Actinobacillus seminis and Histophilus somni infection in rams. BMC Vet. Res. 2013; 9: 51. doi: 10.1186/1746-6148-9-51
19. Burgess, G.W.; McDonald, J.W.; Norris, M.J. Epidemiological studies on ovine brucellosis in selected ram flocks. Aust. Vet. J. 1982; 59:45-47.
20. Paolicchi, F.A.; Casaro, A.P., Gimeno, E.; Kortebani, G.; Mazzolli, A.B. Antisperm response in rams experimentally infected with Brucella ovis. Small Rum. Res. 2000; 36:7-15.
21. Ridler, A.L.; Smith, S.L.; West, D.M. Seroconversion and semen shedding in rams experimentally infected with Brucella ovis. N. Z. Vet. J. 2014; 62:47-50.34.
22. Ward, A.C.; Jaworski, M.D.; Eddow, J.M.; Corbeil, L.B. A comparative study of bovine and ovine Haemophilus somnus isolates. Can. J. Vet. Res. 1995; 59:173–178
23. Appuhamy, S.; Low, J.C.; Parton, R.; Coote, J.G. Specific PCR primers from the 16S–23S rRNA spacer region for the rapid detection and identification of Actinobacillus seminis. J. Appl. Microbiol. 1998; 85:941–948.
24. Saunders, V.F.; Reddacliff, L.A.; Berg, T.; Hornitzky, M. Multiplex PCR for the detection of Brucella ovis, Actinobacillus seminis and Histophilus somni in ram semen. Aust. Vet. J. 2007; 85: 72–77.
25. Foster, R.A.; Ladds, P.W.; Briggs, G.D.; Hoffmann D. Pathology of acessory sex glands of rams infected with Brucella ovis. Aust. Vet. J. 1987; 64: 248-250.
26. Carvalho Júnior, C., Moustacas, V., Xavier, M., Costa, E.A., Costa, L.F., Silva, T.M.A.; Paixão, T.A.; Borges, A.M., Gouvei, A.M.G.; Santos, R.L. Andrological, pathologic, morphometric, and ultrasonographic findings in rams experimentally infected with Brucella ovis. Small Rum. Res. 2012; 102:213-222.
27. Bulgin, M.S. Epididymitis in rams and lambs. Vet. Clin. North. Am. Food Anim. Pract. 1990; 6: 683-690.
28. Robles, C.A.; Uzal, F.A.; Olaechea, F.V., Low, C. Epidemiological observations in a Corriedale flock affected by Brucella ovis. Vet. Res. Commun. 1998; 22:435-443..